http://www.thelancet.com/journals/la...207-6/abstract
The Lancet, Early Online Publication, 24 June 2013
doi:10.1016/S0140-6736(13)61207-6
Copyright © 2013 Elsevier Ltd All rights reserved.
Human infection with avian influenza A H7N9 virus: an assessment of clinical severity
Original Text
The Lancet, Early Online Publication, 24 June 2013
doi:10.1016/S0140-6736(13)61207-6
Copyright © 2013 Elsevier Ltd All rights reserved.
Human infection with avian influenza A H7N9 virus: an assessment of clinical severity
Original Text
Hongjie Yu MD a ?, Benjamin J Cowling PhD e ?, Luzhao Feng MD a ?, Eric HY Lau PhD e ?, Qiaohong Liao MD a, Tim K Tsang MPhil e, Zhibin Peng MD a, Peng Wu PhD e, Fengfeng Liu MD a, Vicky J Fang MPhil e, Honglong Zhang MD a, Ming Li ME a, Lingjia Zeng MSc a, Zhen Xu MD a, Zhongjie Li MD a, Huiming Luo MD b, Qun Li MD c, Zijian Feng MD c, Bin Cao PhD f, Weizhong Yang MD d, Dr Joseph T Wu PhD e, Dr Yu Wang PhD d, Prof Gabriel M Leung MD e
Summary
Background
Characterisation of the severity profile of human infections with influenza viruses of animal origin is a part of pandemic risk assessment, and an important part of the assessment of disease epidemiology. Our objective was to assess the clinical severity of human infections with avian influenza A H7N9 virus, which emerged in China in early 2013.
Methods
We obtained information about laboratory-confirmed cases of avian influenza A H7N9 virus infection reported as of May 28, 2013, from an integrated database built by the Chinese Center for Disease Control and Prevention. We estimated the risk of fatality, mechanical ventilation, and admission to the intensive care unit for patients who required hospital admission for medical reasons. We also used information about laboratory-confirmed cases detected through sentinel influenza-like illness surveillance to estimate the symptomatic case fatality risk.
Findings
Of 123 patients with laboratory-confirmed avian influenza A H7N9 virus infection who were admitted to hospital, 37 (30% had died and 69 (56%) had recovered by May 28, 2013. After we accounted for incomplete data for 17 patients who were still in hospital, we estimated the fatality risk for all ages to be 36% (95% CI 26?45) on admission to hospital. Risks of mechanical ventilation or fatality (69%, 95% CI 60?77) and of admission to an intensive care unit, mechanical ventilation, or fatality (83%, 76?90) were high. With assumptions about coverage of the sentinel surveillance network and health-care-seeking behaviour for patients with influenza-like illness associated with influenza A H7N9 virus infection, and pro-rata extrapolation, we estimated that the symptomatic case fatality risk could be between 160 (63?460) and 2800 (1000?9400) per 100 000 symptomatic cases.
had died and 69 (56%) had recovered by May 28, 2013. After we accounted for incomplete data for 17 patients who were still in hospital, we estimated the fatality risk for all ages to be 36% (95% CI 26?45) on admission to hospital. Risks of mechanical ventilation or fatality (69%, 95% CI 60?77) and of admission to an intensive care unit, mechanical ventilation, or fatality (83%, 76?90) were high. With assumptions about coverage of the sentinel surveillance network and health-care-seeking behaviour for patients with influenza-like illness associated with influenza A H7N9 virus infection, and pro-rata extrapolation, we estimated that the symptomatic case fatality risk could be between 160 (63?460) and 2800 (1000?9400) per 100 000 symptomatic cases.
Interpretation
Human infections with avian influenza A H7N9 virus seem to be less serious than has been previously reported. Many mild cases might already have occurred. Continued vigilance and sustained intensive control efforts are needed to minimise the risk of human infection.
Funding
Chinese Ministry of Science and Technology; Research Fund for the Control of Infectious Disease; Hong Kong University Grants Committee; China?US Collaborative Program on Emerging and Re-emerging Infectious Diseases; Harvard Center for Communicable Disease Dynamics; US National Institute of Allergy and Infectious Disease; and the US National Institutes of Health.
_________
a Division of Infectious Disease, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China; b National Immunization Program, Chinese Center for Disease Control and Prevention, Beijing, China; c Public Health Emergency Center, Chinese Center for Disease Control and Prevention, Beijing, China; d Office of the Director, Chinese Center for Disease Control and Prevention, Beijing, China; e Infectious Disease Epidemiology Group, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; f Department of Infectious Diseases and Clinical Microbiology, Beijing Chao-Yang Hospital, Beijing Institute of Respiratory Medicine, Capital Medical University, Beijing, China
Correspondence to: Dr Joseph T Wu, Infectious Disease Epidemiology Group, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China
Dr Y Wang, Office of the Director, Chinese Center for Disease Control and Prevention, Beijing 102206, China
? Contributed equally
-Summary
Background
Characterisation of the severity profile of human infections with influenza viruses of animal origin is a part of pandemic risk assessment, and an important part of the assessment of disease epidemiology. Our objective was to assess the clinical severity of human infections with avian influenza A H7N9 virus, which emerged in China in early 2013.
Methods
We obtained information about laboratory-confirmed cases of avian influenza A H7N9 virus infection reported as of May 28, 2013, from an integrated database built by the Chinese Center for Disease Control and Prevention. We estimated the risk of fatality, mechanical ventilation, and admission to the intensive care unit for patients who required hospital admission for medical reasons. We also used information about laboratory-confirmed cases detected through sentinel influenza-like illness surveillance to estimate the symptomatic case fatality risk.
Findings
Of 123 patients with laboratory-confirmed avian influenza A H7N9 virus infection who were admitted to hospital, 37 (30%
Interpretation
Human infections with avian influenza A H7N9 virus seem to be less serious than has been previously reported. Many mild cases might already have occurred. Continued vigilance and sustained intensive control efforts are needed to minimise the risk of human infection.
Funding
Chinese Ministry of Science and Technology; Research Fund for the Control of Infectious Disease; Hong Kong University Grants Committee; China?US Collaborative Program on Emerging and Re-emerging Infectious Diseases; Harvard Center for Communicable Disease Dynamics; US National Institute of Allergy and Infectious Disease; and the US National Institutes of Health.
_________
a Division of Infectious Disease, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China; b National Immunization Program, Chinese Center for Disease Control and Prevention, Beijing, China; c Public Health Emergency Center, Chinese Center for Disease Control and Prevention, Beijing, China; d Office of the Director, Chinese Center for Disease Control and Prevention, Beijing, China; e Infectious Disease Epidemiology Group, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; f Department of Infectious Diseases and Clinical Microbiology, Beijing Chao-Yang Hospital, Beijing Institute of Respiratory Medicine, Capital Medical University, Beijing, China
Correspondence to: Dr Joseph T Wu, Infectious Disease Epidemiology Group, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China
Dr Y Wang, Office of the Director, Chinese Center for Disease Control and Prevention, Beijing 102206, China
? Contributed equally
-------
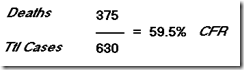

Comment