Please remember there is a time lag of about 2 weeks in the above data due to standard collection procedures.
Announcement
Collapse
No announcement yet.
US FluView: 2017-2018 season
Collapse
X
-
Since Week 1, the MMWRs do not contain the table of notifiable diseases, which lists the number of pediatric influenza deaths in each state. I cannot find this information elsewhere. If anyone can locate the tables for Weeks 1 and 2, please post a link here."I know God will not give me anything I can't handle. I just wish that He didn't trust me so much." - Mother Teresa of Calcutta
Comment
-
2017-2018 Influenza Season Week 3 ending January 20, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 3 (January 14-20, 2018), influenza activity increased in the United States.- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 3 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories slightly increased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Seven influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 41.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 6.6%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, Puerto Rico, and 39 states experienced high ILI activity; the District of Columbia and five states experienced moderate ILI activity; three states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread; Guam reported regional activity; the District of Columbia and one state reported local activity; and the U.S. Virgin Islands reported sporadic activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 51 of 54 26.7% 1,530 15,376 299 184 1,750 730 37 Elevated 6 of 6 20.2% 54 657 2 4 85 1 0 Elevated 3 of 4 20.7% 58 625 5 1 68 46 2 Elevated 5 of 6 23.2% 297 1,351 2 20 244 15 1 Elevated 8 of 8 25.2% 259 1,076 63 4 95 124 7 Elevated 6 of 6 27.5% 217 3,354 34 21 245 47 5 Elevated 5 of 5 30.5% 190 755 16 2 117 78 7 Elevated 4 of 4 24.8% 27 763 19 0 169 5 0 Elevated 6 of 6 22.3% 74 1,405 14 10 211 8 1 Elevated 4 of 5 23.9% 228 4,550 130 117 322 296 12 Elevated 4 of 4 27.5% 126 840 14 5 194 110 2
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
? Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
50,276 513,252 13,421 (26.7%) 83,450 (16.3%) 10,536 (78.5%) 68,517 (82.1%) 2,885 (21.5%) 14,933 (17.9%) 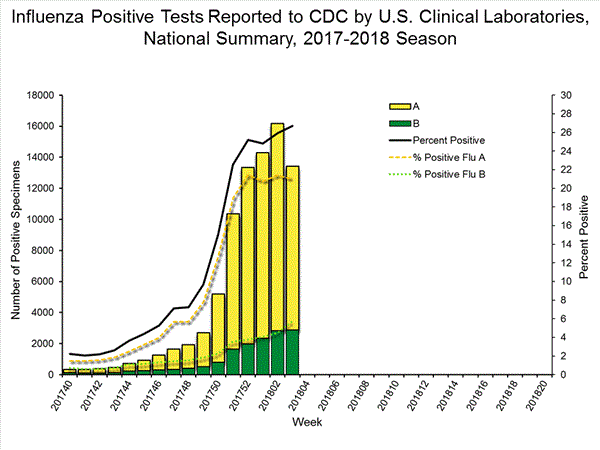
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.2,209 39,400 1,349 19,869 1,136 (84.2%) 17,205 (86.6%) 144 (12.7%) 1,530 (8.9%) 914 (80.5%) 15,376 (89.4%) 78 (6.9%) 299 (1.7%) 213 (15.8%) 2,664 (13.2%) 127 (59.6%) 1,750 (65.7%) 8 (3.8%) 184 (6.9%) 78 (36.6%) 730 (27.4%)
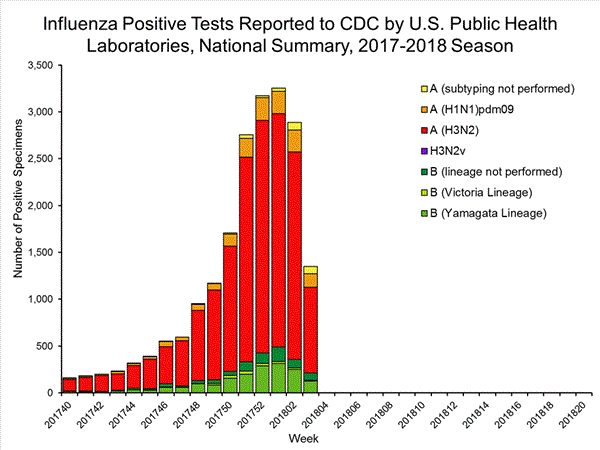
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation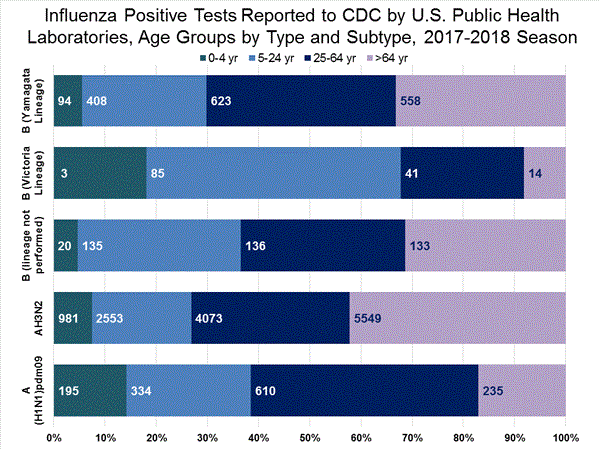
View Interactive Application | View Full Screen
Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) (i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 1,041 influenza viruses collected during October 1, 2017 ? January 20, 2018, and submitted by U.S. laboratories, including 181 influenza A(H1N1)pdm09 viruses, 561 influenza A(H3N2) viruses, and 299 influenza B viruses.- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 181 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Eighty-five A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017?18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 561 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=461), subclade 3C.2a1 (n=93) or clade 3C.3a (n=7). One hundred ninety four influenza A(H3N2) viruses were antigenically characterized, and 191 (98.5%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017?18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 43 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a small number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Sixteen (59.3%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017?18 Northern Hemisphere influenza vaccines. Eleven (40.7%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 256 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 152 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017?18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmapconsiderations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week.. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
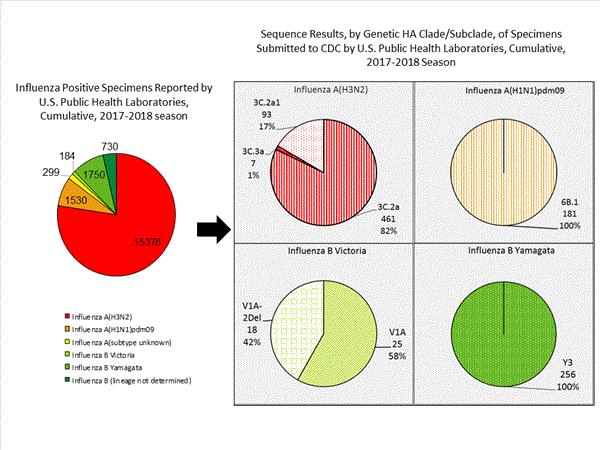
View Chart Data | View Full Screen | View PowerPoint Presentation
Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.1812 (1.1)1470 (0.0)1812 (1.1)6450 (0.0)6450 (0.0)4740 (0.0)2290 (0.0)2290 (0.0)2290 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
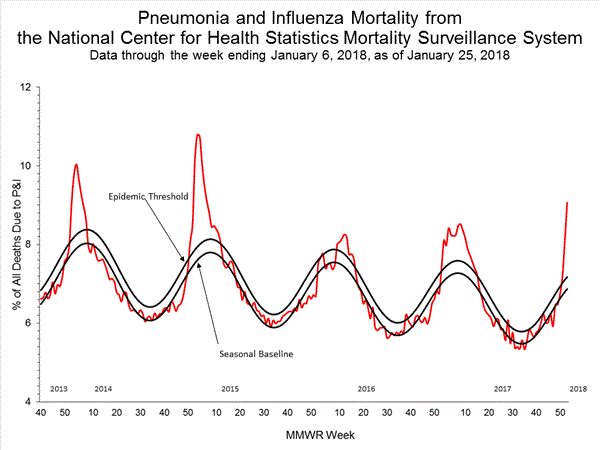
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on January 25, 2018, 9.1% of the deaths occurring during the week ending January 6, 2018 (week 1) were due to P&I. This percentage is above the epidemic threshold of 7.2% for week 1.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. Due to the additional time needed for manual coding, the initially reported P&I percentages may be lower than percentages calculated from final data. Previous longer backlogs in manual coding have been resolved and death records are now coded within 10 days from receipt of a death record by NCHS.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
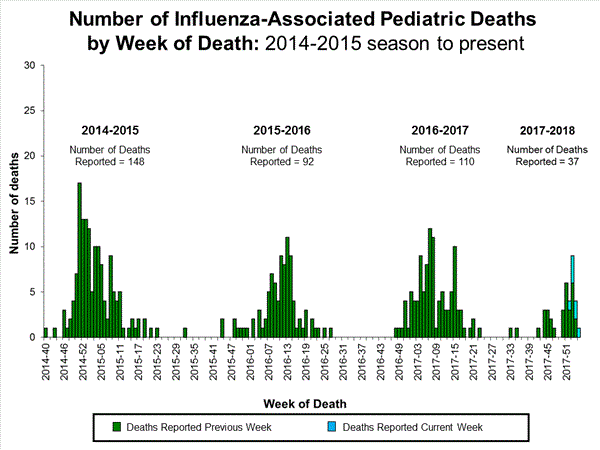
Influenza-Associated Pediatric Mortality:
Seven influenza-associated pediatric deaths were reported to CDC during week 3. One death was associated with an influenza A(H3) virus and occurred during week 2 (the week ending January 13, 2018). Two deaths were associated with an influenza A(H1N1)pdm09 virus and occurred during weeks 1 and 3 (the weeks ending January 6, 2018, and January 20, 2018, respectively). Three deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 52 and 1 (the weeks ending December 30, 2017, and January 6, 2018, respectively). One death was associated with an influenza B virus and occurred during week 2.
A total of 37 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 11,965 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and January 20, 2018. The overall hospitalization rate was 41.9 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (183.1 per 100,000 population), followed by adults aged 50-64 (44.2 per 100,000 population) and children aged 0-4 years (27.0 per 100,000 population). Among 11,965 hospitalizations, 10,612 (88.7%) were associated with influenza A virus, 1,295 (10.8%) with influenza B virus, 28 (0.2%) with influenza A virus and influenza B virus co-infection, and 30 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 2,360 (86.4%) were A(H3N2) and 372 (13.6%) were A(H1N1)pdm09 virus.
Among 1,445 hospitalized adults with information on underlying medical conditions, 1,038 (71.8%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 148 hospitalized children with information on underlying medical conditions, 83 (56.1%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 115 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 29 (25.2%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics? (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation
FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications;Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
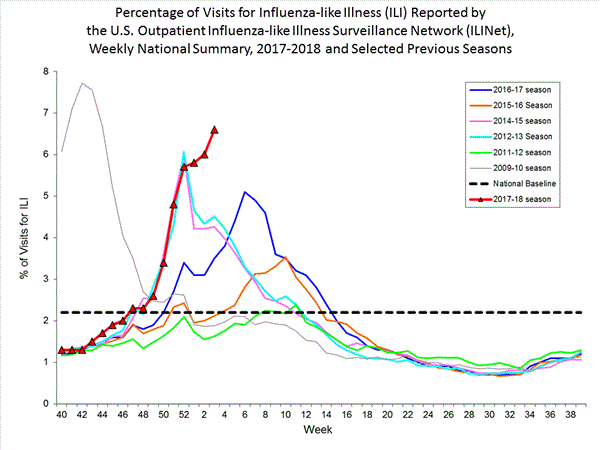
Outpatient Illness Surveillance:
Nationwide during week 3, 6.6% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100?F [37.8?C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 2.9% to 11.7% during week 3. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 3, the following ILI activity levels were experienced:- New York City, Puerto Rico, and 39 states experienced high activity (Alabama, Arizona, Arkansas, California, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
- The District of Columbia and five states experienced moderate ILI activity (Colorado, Connecticut, Hawaii, Idaho, and Vermont).
- Three states experienced low ILI activity (Alaska, North Dakota and Utah).
- Three states experienced minimal ILI activity (Delaware, Maine, and Montana).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 3, the following influenza activity was reported::- Widespread influenza activity was reported by Puerto Rico and 49 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
- Regional influenza activity was reported by Guam.
- Local influenza activity was reported by the District of Columbia and one state (Hawaii).
- Sporadic activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available athttps://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
Comment
-
2017-2018 Influenza Season Week 4 ending January 27, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 4 (January 21-27, 2018), influenza activity increased in the United States.- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 4 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories remained elevated.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Seventeen influenza-associated pediatric deaths were reported, one of which occurred during the 2015-2016 season.
- Influenza-associated Hospitalizations: A cumulative rate of 51.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 7.1%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, the District of Columbia, and 42 states experienced high ILI activity; Puerto Rico and two states experienced moderate ILI activity; three states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 48 states was reported as widespread; Guam and one state reported regional activity; the District of Columbia and one state reported local activity; and the U.S. Virgin Islands reported sporadic activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 51 of 54 26.1% 1,896 18,068 348 228 2,292 921 53 Elevated 6 of 6 24.0% 74 939 2 5 135 8 1 Elevated 3 of 4 23.5% 78 731 5 1 78 56 2 Elevated 5 of 6 24.6% 354 1,645 9 28 313 17 2 Elevated 8 of 8 24.6% 315 1,314 88 7 142 171 14 Elevated 6 of 6 30.3% 281 3,928 44 27 359 17 8 Elevated 5 of 5 32.3% 216 840 16 2 150 110 9 Elevated 4 of 4 23.7% 36 863 10 1 207 7 1 Elevated 6 of 6 21.0% 106 1,669 14 15 267 8 1 Elevated 4 of 5 18.3% 268 5,158 150 135 413 344 13 Elevated 4 of 4 24.6% 168 981 10 7 228 183 2
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
? Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
59,200 584,362 15,427 (26.1%) 102,364 (17.5%) 11,792 (76.4%) 83,239 (81.3%) 3,635 (23.6%) 19,125 (18.7%) 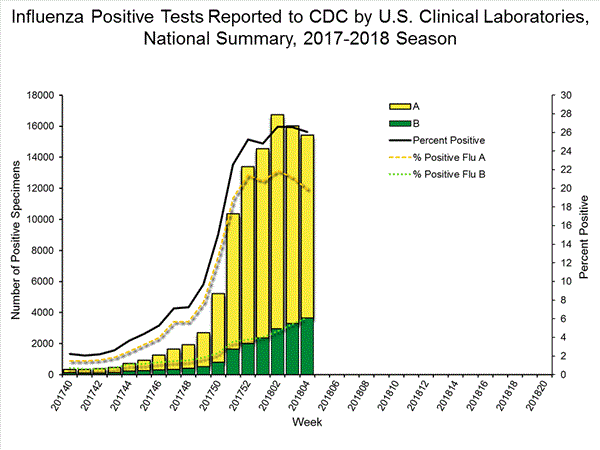
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.2,660 44,852 1,597 23,753 1,280(80.2%) 20,312 (85.5%) 189 (14.8%) 1,896 (9.3%) 1,017 (79.5%) 18,068 (89.0%) 74 (5.8%) 348 (1.7%) 317 (19.8%) 3,441 (14.5%) 201 (63.4%) 2,292 (66.6%) 13 (4.1%) 228 (6.6%) 103 (32.5%) 921 (26.8%)
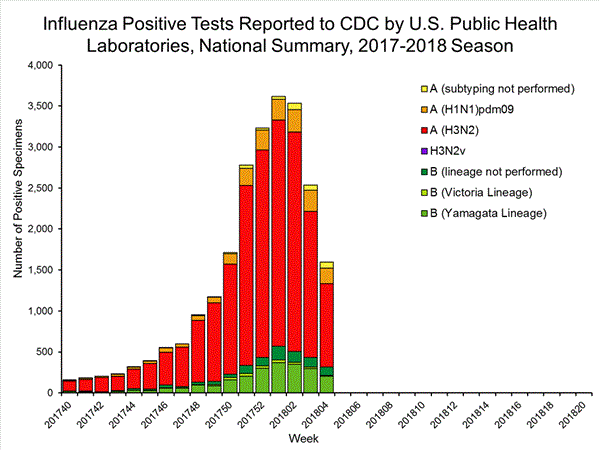
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation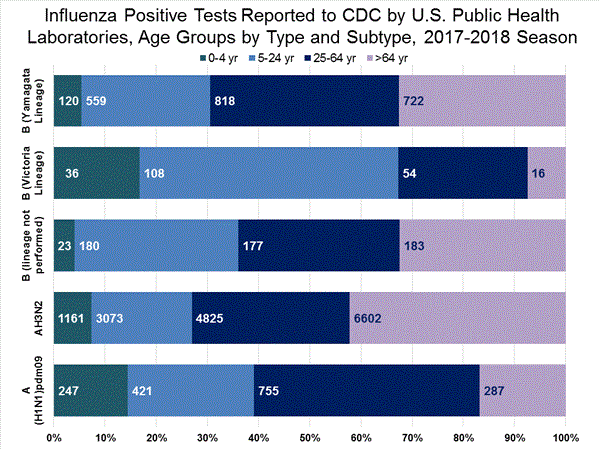
View Interactive Application | View Full Screen Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) (i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 1,289 influenza viruses collected during October 1, 2017 ? January 27, 2018, and submitted by U.S. laboratories, including 253 influenza A(H1N1)pdm09 viruses, 668 influenza A(H3N2) viruses, and 368 influenza B viruses.- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 253 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. One hundred thirty six A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017?18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 668 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=554), subclade 3C.2a1 (n=104) or clade 3C.3a (n=10). Two hundred forty five influenza A(H3N2) viruses were antigenically characterized, and 242 (98.8%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017?18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 51 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Seventeen (58.6%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017?18 Northern Hemisphere influenza vaccines. Twelve (41.4%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 317 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 202 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017?18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmapconsiderations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week.. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
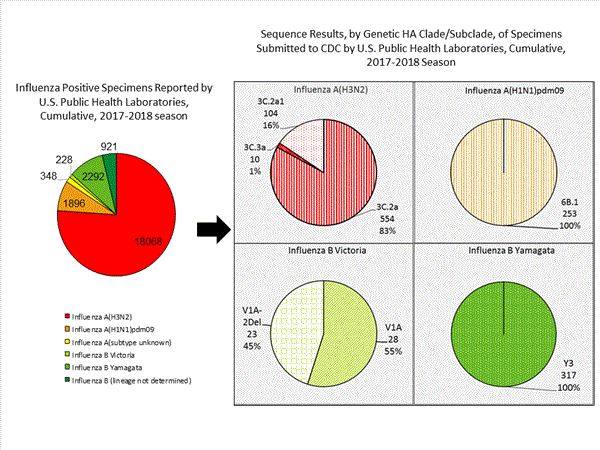
View Chart Data | View Full Screen | View PowerPoint Presentation Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.2822 (0.7)2350 (0.0)2822 (0.7)8280 (0.0)8280 (0.0)6100 (0.0)3370 (0.0)3370 (0.0)3370 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on February 1, 2018, 9.7% of the deaths occurring during the week ending January 13, 2018 (week 2) were due to P&I. This percentage is above the epidemic threshold of 7.2% for week 2.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
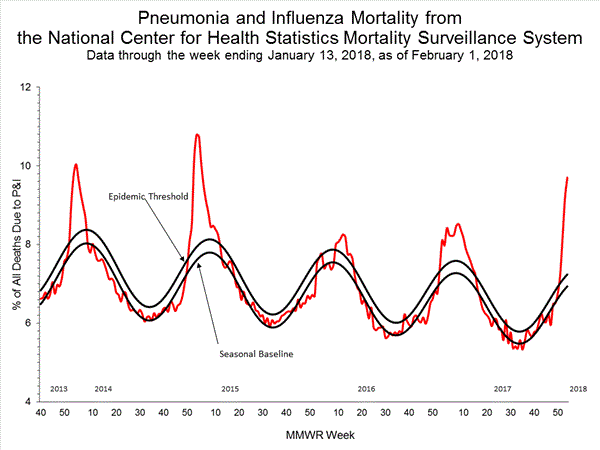
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Seventeen influenza-associated pediatric deaths were reported to CDC during week 4.
Five deaths were associated with an influenza A(H3) virus and occurred during weeks 1, 2, 3, and 4 (the weeks ending January 6, January 13, January 20, and January 27, 2018). Two deaths were associated with an influenza A(H1N1)pdm09 virus and occurred during weeks 3 and 4 (the weeks ending January 20, 2018, and January 27, 2018, respectively). Four deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 3 and 4. Five deaths were associated with an influenza B virus and occurred during weeks 1, 3, and 4 (the week ending January 6, January 20, and January 27, 2018, respectively).
A total of 53 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
One death that occurred during the 2015-2016 season was associated with an influenza A virus for which no subtyping was performed and occurred during week 28 (the week ending July 16, 2016). This death brings the total number of reported influenza-associated deaths occurring during that season to 93.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
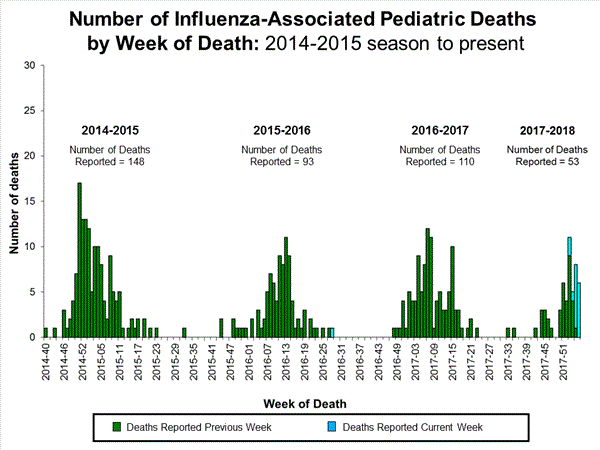
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 14,676 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and January 27, 2018. The overall hospitalization rate was 51.4 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (226.8 per 100,000 population), followed by adults aged 50-64 (54.0 per 100,000 population) and children aged 0-4 years (33.3 per 100,000 population). Among 14,676 hospitalizations, 12,849 (87.5%) were associated with influenza A virus, 1,762 (12.0%) with influenza B virus, 35 (0.2%) with influenza A virus and influenza B virus co-infection, and 30 (0.2%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 2,797 (86.5%) were A(H3N2) and 437 (13.5%) were A(H1N1)pdm09 virus.
Among 1,708 hospitalized adults with information on underlying medical conditions, 1,183 (69.3%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 180 hospitalized children with information on underlying medical conditions, 93 (51.7%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 138 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 33 (23.9%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics? (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications;Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 4, 7.1% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100?F [37.8?C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
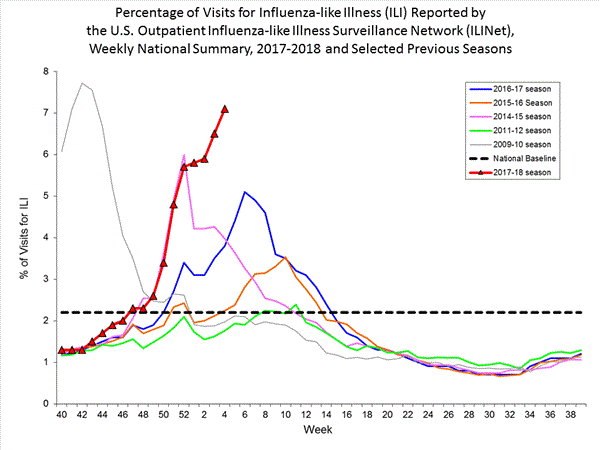
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation On a regional level, the percentage of outpatient visits for ILI ranged from 2.8% to 13.0% during week 4. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 4, the following ILI activity levels were experienced:- New York City, the District of Columbia, and 42 states experienced high activity (Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Florida, Georgia, Hawaii, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Vermont, Virginia, West Virginia, Wisconsin, and Wyoming).
- Puerto Rico and two states experienced moderate ILI activity (California and Idaho).
- Three states experienced low ILI activity (Delaware, North Dakota and Washington).
- Three states experienced minimal ILI activity (Maine, Montana, and Utah).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 4, the following influenza activity was reported::- Widespread influenza activity was reported by Puerto Rico and 48 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
- Regional influenza activity was reported by Guam and one state (Oregon).
- Local influenza activity was reported by the District of Columbia and one state (Hawaii).
- Sporadic activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available athttps://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
--------------------------------------------------------------------------------
File Formats Help:
How do I view different file formats (PDF, DOC, PPT, MPEG) on this site?
[/COLOR]
- Page last reviewed: February 2, 2018
Comment
-
2017-2018 Influenza Season Week 5 ending February 3, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 5 (January 28-February 3, 2018), influenza activity increased in the United States.- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 5 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories remained elevated.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Ten influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 59.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, the District of Columbia, Puerto Rico and 43 states experienced high ILI activity; three states experienced moderate ILI activity; two states experienced low ILI activity; and two states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 48 states was reported as widespread; two states reported regional activity; the District of Columbia and Guam reported local activity; and the U.S. Virgin Islands reported sporadic activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 51 of 54 26.3% 2,298 20,512 445 309 3,010 1,093 63 Elevated 6 of 6 26.6% 118 1,159 2 7 200 10 1 Elevated 3 of 4 28.8% 108 931 5 5 127 79 2 Elevated 5 of 6 25.4% 418 1,928 10 41 393 14 3 Elevated 8 of 8 25.8% 395 1,660 133 10 202 215 18 Elevated 6 of 6 30.6% 315 4,379 44 27 442 18 10 Elevated 5 of 5 29.1% 233 898 16 2 202 127 9 Elevated 4 of 4 21.6% 44 924 14 1 250 5 1 Elevated 6 of 6 20.1% 128 1,864 15 19 330 8 1 Elevated 4 of 5 15.2% 345 5,699 195 190 589 408 15 Elevated 4 of 4 21.3% 194 1,070 11 7 275 209 3
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
? Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
63,180 666,493 16,641 (26.3%) 124,316 (18.7%) 11,517 (69.2%) 98,606 (79.3%) 5,124 (30.8%) 25,710 (20.7%) 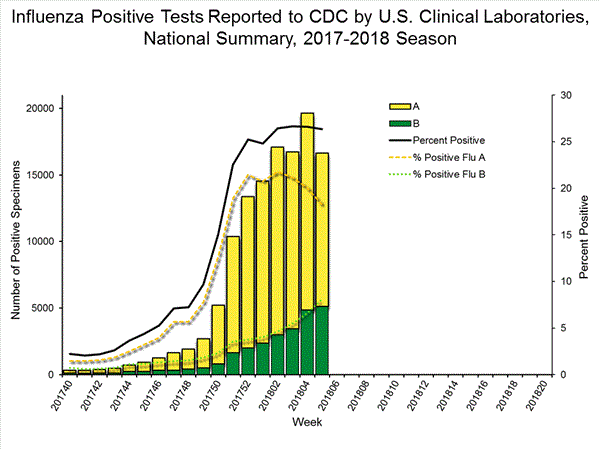
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.2,608 51,014 1,453 27,667 1,065 (73.3%) 23,255 (84.1%) 144 (13.5%) 2,298 (9.9%) 834 (78.3%) 20,512 (88.2%) 87 (8.2%) 445 (1.9%) 388 (26.7%) 4,412 (15.9%) 266 (68.6%) 3,010 (68.2%) 22 (5.7%) 309 (7.0%) 100 (25.8%) 1,093 (24.8%)
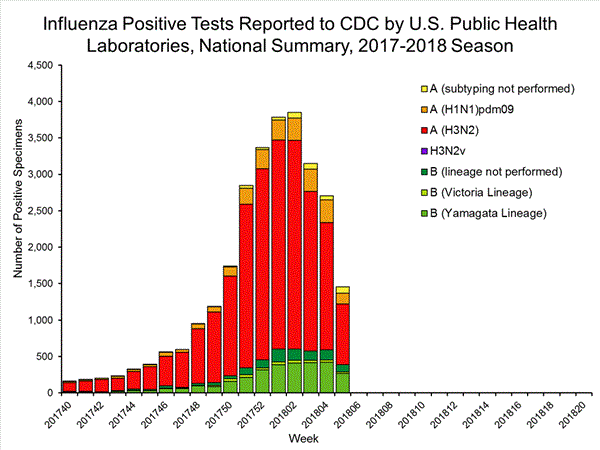
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation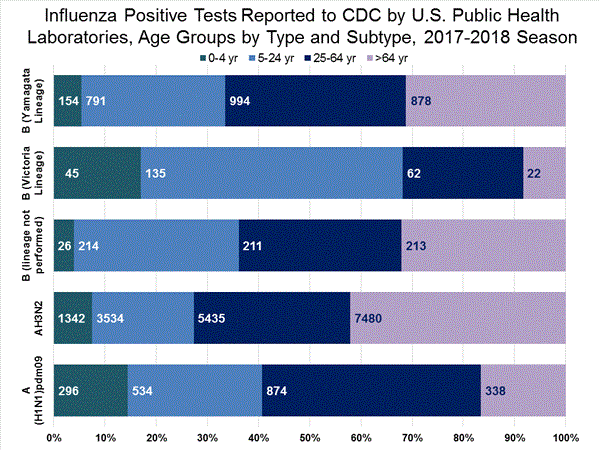
View Interactive Application | View Full Screen Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) (i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 1,365 influenza viruses collected during October 1, 2017 ? February 3, 2018, and submitted by U.S. laboratories, including 276 influenza A(H1N1)pdm09 viruses, 695 influenza A(H3N2) viruses, and 394 influenza B viruses.- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 276 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Two hundred five A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017?18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 695 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=577), subclade 3C.2a1 (n=108) or clade 3C.3a (n=10). Two hundred sixty-two influenza A(H3N2) viruses were antigenically characterized, and 257 (98.1%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017?18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 56 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Seventeen (58.6%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017?18 Northern Hemisphere influenza vaccines. Twelve (41.4%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 338 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 202 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017?18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmapconsiderations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week.. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
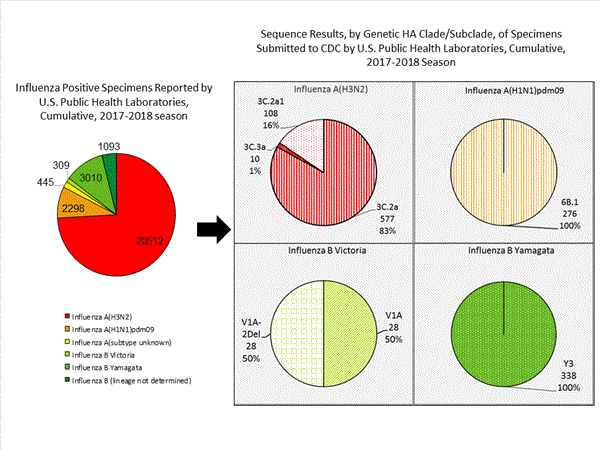
View Chart Data | View Full Screen | View PowerPoint Presentation Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.3764 (1.1)2650 (0.0)3764 (1.1)9030 (0.0)9030 (0.0)6380 (0.0)3870 (0.0)3870 (0.0)3870 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on February 8, 2018, 10.1% of the deaths occurring during the week ending January 20, 2018 (week 3) were due to P&I. This percentage is above the epidemic threshold of 7.3% for week 3.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
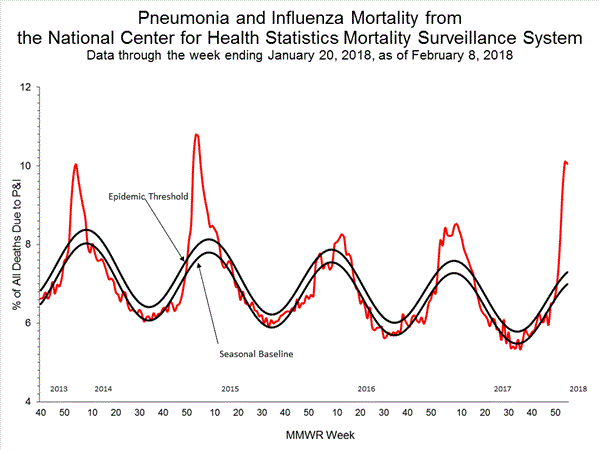
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Ten influenza-associated pediatric deaths were reported to CDC during week 5. Four deaths were associated with an influenza A(H3) virus and occurred during weeks 3 and 4 (the weeks ending January 20, and January 27, 2018, respectively). Three deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 52, 4, and 5 (the weeks ending December 30, 2017, January 27, and February 3, 2018, respectively). Three deaths were associated with an influenza B virus and occurred during weeks 2 and 4 (the weeks ending January 13, and January 27, 2018, respectively).
A total of 63 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
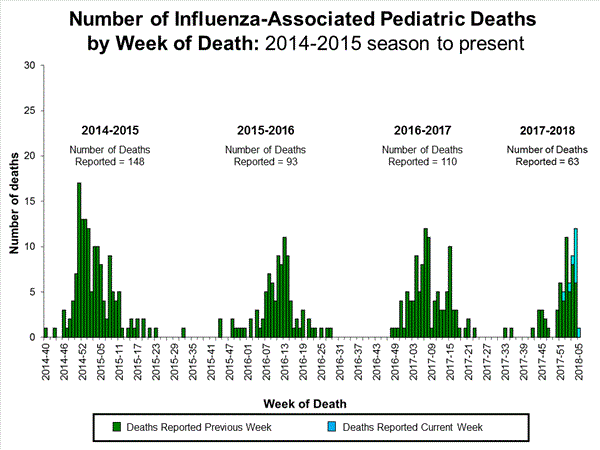
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 17,101 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and February 3, 2018. The overall hospitalization rate was 59.9 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (263.6 per 100,000 population), followed by adults aged 50-64 (63.1 per 100,000 population) and children aged 0-4 years (40.0 per 100,000 population). Among 17,101 hospitalizations, 14,770 (86.4%) were associated with influenza A virus, 2,251 (13.2%) with influenza B virus, 43 (0.3%) with influenza A virus and influenza B virus co-infection, and 37 (0.2%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 3,308 (86.1%) were A(H3N2) and 533 (13.9%) were A(H1N1)pdm09 virus.
Among 1,955 hospitalized adults with information on underlying medical conditions, 1,325 (67.8%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 192 hospitalized children with information on underlying medical conditions, 97 (50.5%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 151 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 36 (23.8%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics? (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications;Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 5, 7.7% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100?F [37.8?C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
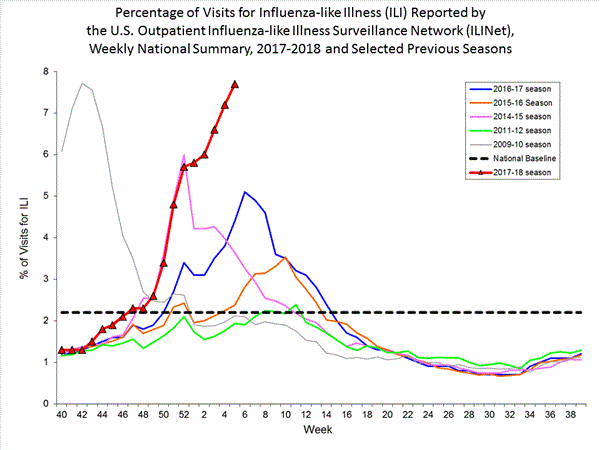
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation On a regional level, the percentage of outpatient visits for ILI ranged from 3.4% to 12.5% during week 5. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 5, the following ILI activity levels were experienced:- New York City, the District of Columbia, Puerto Rico and 43 states experienced high activity (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Vermont, Virginia, West Virginia, Wisconsin, and Wyoming).
- Three states experienced moderate ILI activity (Hawaii, Idaho, and Washington).
- Two states experienced low ILI activity (North Dakota and Utah).
- Two states experienced minimal ILI activity (Maine and Montana).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 5, the following influenza activity was reported:- Widespread influenza activity was reported by Puerto Rico and 48 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
- Regional influenza activity was reported by two states (Hawaii and Oregon).
- Local influenza activity was reported by the District of Columbia and Guam.
- Sporadic activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available athttps://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
--------------------------------------------------------------------------------
File Formats Help:
How do I view different file formats (PDF, DOC, PPT, MPEG) on this site?
[/COLOR]
- Page last reviewed: February 9, 2018
Comment
-
2017-2018 Influenza Season Week 6 ending February 10, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 6 (February 4-10, 2018), influenza activity remained elevated in the United States.- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 6 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories remained elevated.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Twenty-two influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 67.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 7.5%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, the District of Columbia, Puerto Rico and 43 states experienced high ILI activity; two states experienced moderate ILI activity; three states experienced low ILI activity; and two states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 48 states was reported as widespread; one state reported regional activity; the District of Columbia, Guam and one state reported local activity; and the U.S. Virgin Islands reported no activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 50 of 54 26.5% 3,018 23,775 420 399 3,808 1,710 84 Elevated 6 of 6 30.1% 142 1,289 2 14 242 15 1 Elevated 3 of 4 31.4% 173 1,162 8 6 142 169 5 Elevated 5 of 6 25.5% 524 2,344 11 67 488 43 6 Elevated 8 of 8 24.4% 559 2,188 103 14 290 362 24 Elevated 6 of 6 30.1% 444 5,104 64 29 599 86 16 Elevated 5 of 5 28.1% 292 1,120 24 2 240 235 11 Elevated 4 of 4 21.8% 56 1,020 18 1 287 26 2 Elevated 6 of 6 19.1% 175 2,155 15 33 410 37 1 Elevated 3 of 5 15.0% 423 6,206 162 225 788 465 15 Elevated 4 of 4 19.3% 230 1,187 13 8 322 272 3
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
? Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
64,312 744,661 17,040 (26.5%) 144,910 (19.5%) 10,837 (63.6%) 111,947 (77.3%) 6,203 (36.4%) 32,963 (22.7%) 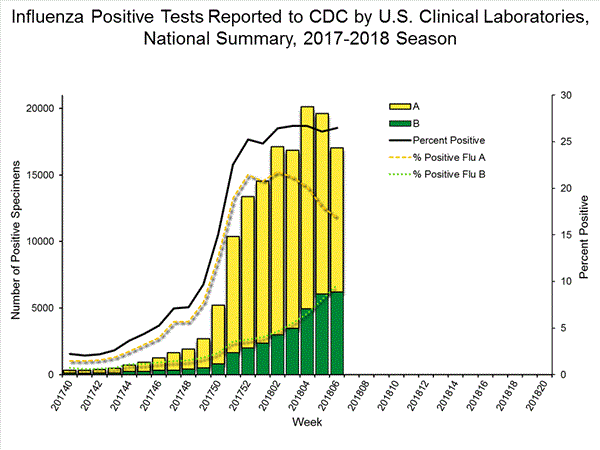
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.2,937 60,372 1,676 33,130 1,109 (66.2%) 27,213 (82.1%) 201 (18.1%) 3,018 (11.1%) 870 (78.4%) 23,775 (87.4%) 38 (3.4%) 420 (1.5%) 567 (33.8%) 5,917 (17.9%) 340 (60.0%) 3,808 (64.4%) 26 (4.6%) 399 (6.7%) 201 (35.4%) 1,710 (28.9%)
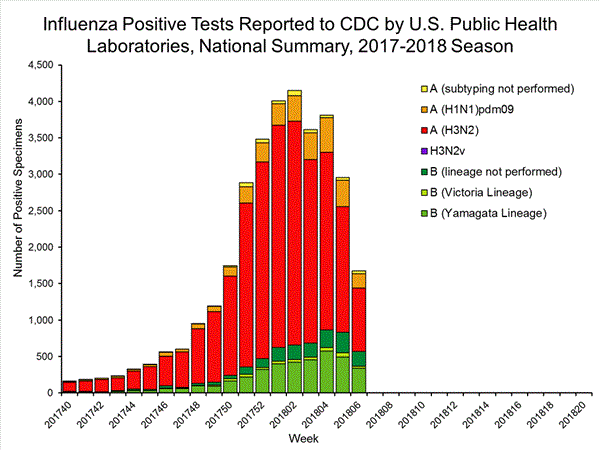
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation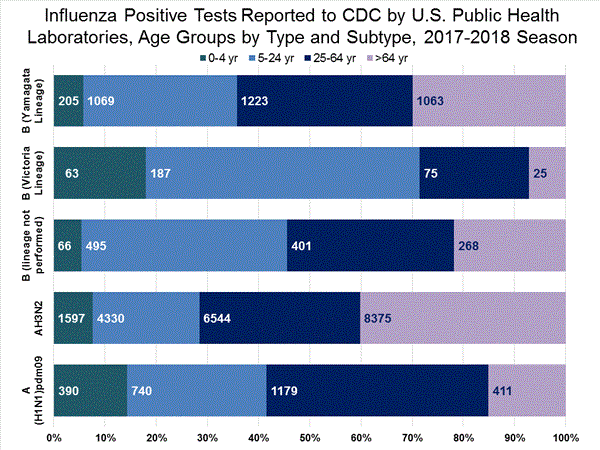
View Interactive Application | View Full Screen Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) ((i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination. On February 15, 2018, interim influenza vaccine effectiveness estimates for the 2017-2018 season were released and are available here.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 1,562 influenza viruses collected during October 1, 2017 ? February 10, 2018, and submitted by U.S. laboratories, including 337 influenza A(H1N1)pdm09 viruses, 769 influenza A(H3N2) viruses, and 456 influenza B viruses.- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 337 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Two hundred and five A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017?18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 769 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=645), subclade 3C.2a1 (n=113) or clade 3C.3a (n=11). Two hundred ninety seven influenza A(H3N2) viruses were antigenically characterized, and 291 (98.0%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017?18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 65 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Twenty-eight (54.9%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017?18 Northern Hemisphere influenza vaccines. Twenty-three (45.1%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 391 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 202 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017?18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmapconsiderations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week.. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
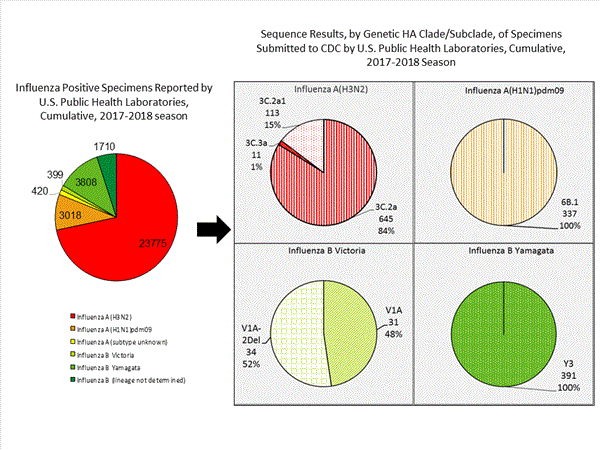
View Chart Data | View Full Screen | View PowerPoint Presentation Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.4314 (0.9)3090 (0.0)4314 (0.9)9620 (0.0)9620 (0.0)6940 (0.0)4180 (0.0)4180 (0.0)4180 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on February 15, 2018, 9.8% of the deaths occurring during the week ending January 27, 2018 (week 4) were due to P&I. This percentage is above the epidemic threshold of 7.3% for week 4.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
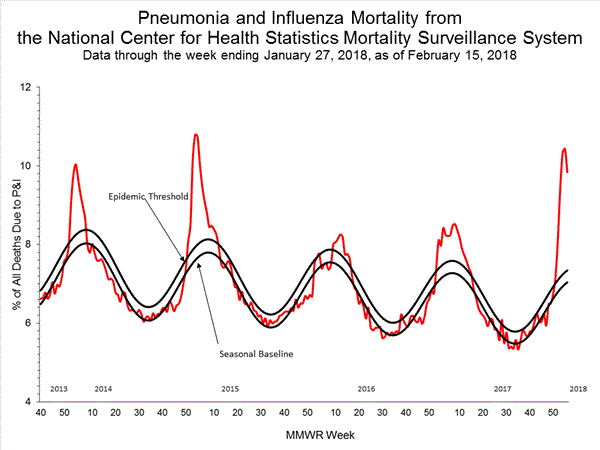
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Twenty-two influenza-associated pediatric deaths were reported to CDC during week 6. Four deaths were associated with an influenza A(H3) virus and occurred during weeks 2, 4, and 5 (the weeks ending January 13, January 27, and February 3, 2018, respectively). Five deaths were associated with an influenza A(H1N1)pdm09 virus and occurred during weeks 52, 5, and 6 (the weeks ending December 30, 2017, February 3, and February 10, 2018, respectively). Eight deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 51, 2, 3, 5, and 6 (the weeks ending December 23, 2017, January 13, January 20, February 3, and February 10, 2018, respectively). Five deaths were associated with an influenza B virus and occurred during weeks 2, 5 and 6 (the weeks ending January 13, February 3, and February 10, 2018, respectively).
One death that was reported earlier this season was reclassified by the reporting jurisdiction. A total of 84 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
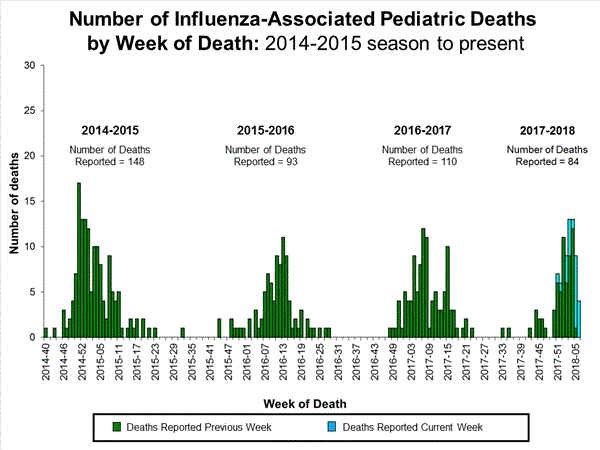
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 19,398 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and February 10, 2018. The overall hospitalization rate was 67.9 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (294.9 per 100,000 population), followed by adults aged 50-64 (72.8 per 100,000 population) and children aged 0-4 years (47.1 per 100,000 population). Among 19,398 hospitalizations, 16,479 (85.0%) were associated with influenza A virus, 2,817 (14.5%) with influenza B virus, 54 (0.3%) with influenza A virus and influenza B virus co-infection, and 48 (0.2%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 3,686 (86.2%) were A(H3N2) and 592 (13.8%) were A(H1N1)pdm09 virus.
Among 2,261 hospitalized adults with information on underlying medical conditions, 1,521 (67.3%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 211 hospitalized children with information on underlying medical conditions, 103 (48.8%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 170 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 40 (23.5%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics? (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications;Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 6, 7.5% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100?F [37.8?C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
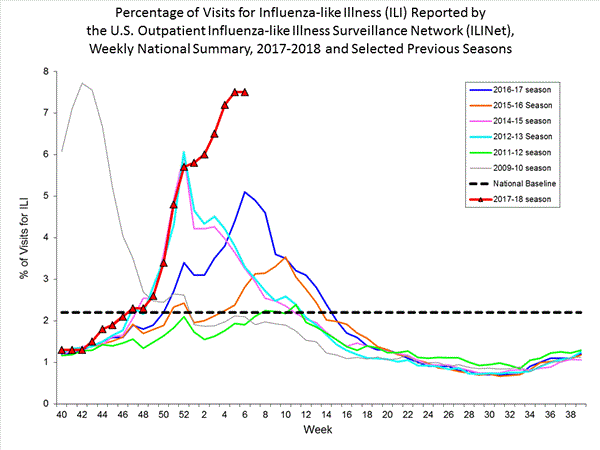
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation On a regional level, the percentage of outpatient visits for ILI ranged from 3.0% to 11.8% during week 6. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 6, the following ILI activity levels were experienced:- New York City, the District of Columbia, Puerto Rico and 43 states experienced high activity (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Vermont, Virginia, West Virginia, Wisconsin, and Wyoming).
- Two states experienced moderate ILI activity (North Dakota and Utah).
- Three states experienced low ILI activity (Hawaii, Idaho, and Washington).
- Two states experienced minimal ILI activity (Maine and Montana).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 6, the following influenza activity was reported:- Widespread influenza activity was reported by Puerto Rico and 48 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
- Regional influenza activity was reported by one state (Oregon).
- Local influenza activity was reported by the District of Columbia, Guam and one state (Hawaii).
- No influenza activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available athttps://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
--------------------------------------------------------------------------------
File Formats Help:
How do I view different file formats (PDF, DOC, PPT, MPEG) on this site?
[/COLOR]
- Page last reviewed: February 16, 2018
Comment
-
2017-2018 Influenza Season Week 8 ending February 24, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 8 (February 18-24, 2018), influenza activity decreased in the United States.- Viral Surveillance: While influenza A(H3) viruses continue to be predominant this season, during week 8 the overall proportion of influenza A viruses is declining and the proportion of influenza B viruses is increasing. The percentage of respiratory specimens testing positive for influenza in clinical laboratories decreased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Seventeen influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 81.7 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 5.0%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, the District of Columbia, and 32 states experienced high ILI activity; Puerto Rico and nine states experienced moderate ILI activity; six states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 45 states was reported as widespread; Guam and two states reported regional activity; the District of Columbia and three states reported local activity; and the U.S. Virgin Islands reported no activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 49 of 54 21.6% 3,825 27,096 369 552 5,189 2,320 114 Elevated 5 of 6 31.9% 220 1,611 2 30 378 21 4 Elevated 3 of 4 31.1% 228 1,408 9 11 195 234 8 Elevated 5 of 6 26.9% 681 2,786 11 109 702 81 8 Elevated 8 of 8 21.5% 715 2,580 87 25 444 471 33 Elevated 6 of 6 29.0% 596 5,948 69 41 840 141 22 Elevated 5 of 5 24.1% 330 1,253 7 2 304 330 16 Elevated 4 of 4 22.1% 66 1,107 10 2 359 32 2 Elevated 6 of 6 17.8% 220 2,427 15 62 574 52 1 Elevated 4 of 5 13.7% 502 6,679 146 261 986 606 17 Elevated 3 of 4 16.9% 267 1,297 13 9 407 352 3
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
? Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
42,615 879,193 9,191 (21.6%) 178,241 (20.3%) 4,742 (51.6%) 131,642 (73.9%) 4,449 (48.4%) 46,599 (26.1%) 
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.1,996 70,369 1,095 39,351 593 (54.2%) 31,290 (79.5%) 115 (19.4%) 3,825 (12.2%) 467 (78.8%) 27,096 (86.6%) 11 (1.9%) 369 (1.2%) 502 (45.8%) 8,061 (20.5%) 322 (64.1%) 5,189 (64.4%) 36 (7.2%) 552 (6.8%) 144 (28.7%) 2,320 (28.8%)
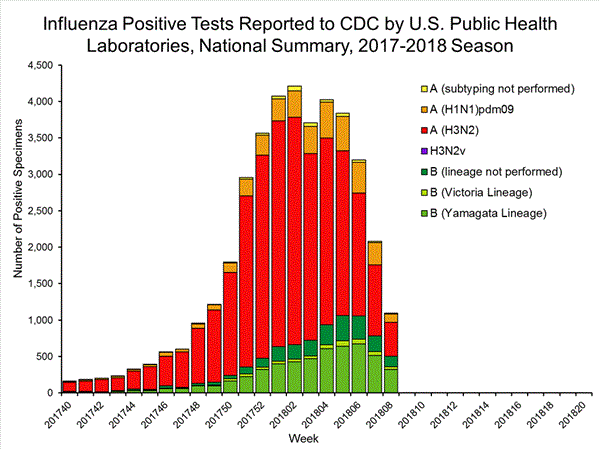
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation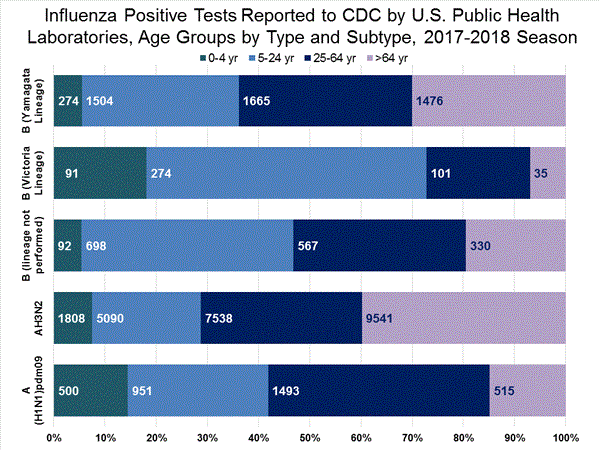
View Interactive Application | View Full Screen Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) ((i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination. On February 15, 2018, interim influenza vaccine effectiveness estimates for the 2017-2018 season were released and are available here.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 1,802 influenza viruses collected during October 1, 2017 ? February 24, 2018, and submitted by U.S. laboratories, including 421 influenza A(H1N1)pdm09 viruses, 859 influenza A(H3N2) viruses, and 522 influenza B viruses.Influenza A Viruses- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 421 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Three hundred thirteen A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017?18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 859 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=726), subclade 3C.2a1 (n=120) or clade 3C.3a (n=13). Three hundred eighty-one influenza A(H3N2) viruses were antigenically characterized, and 373 (97.9%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017?18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 77 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Thirty-one (44.3%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017?18 Northern Hemisphere influenza vaccines. Thirty-nine (55.7%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 445 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 319 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017?18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmapconsiderations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.

View Chart Data | View Full Screen | View PowerPoint Presentation Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.5205 (1.0)3800 (0.0)5205 (1.0)1,1170 (0.0)1,1170 (0.0)7840 (0.0)4820 (0.0)4820 (0.0)4820 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on March 1, 2018, 9.0% of the deaths occurring during the week ending February 10, 2018 (week 6) were due to P&I. This percentage is above the epidemic threshold of 7.4% for week 6.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
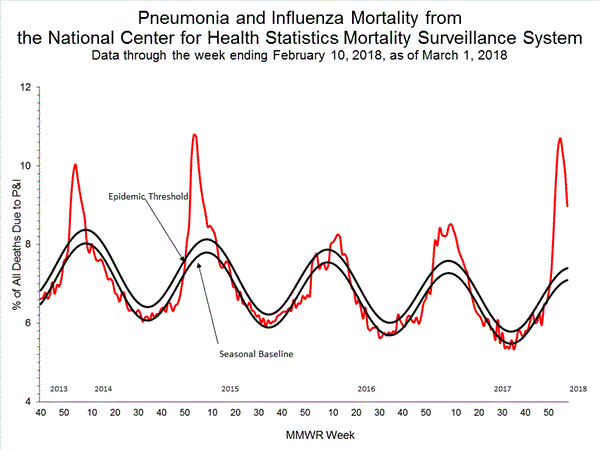
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Seventeen influenza-associated pediatric deaths were reported to CDC during week 8. Three deaths were associated with an influenza A(H3) virus and occurred during weeks 7 and 8 (the weeks ending February 17 and February 24, 2018, respectively). One death was associated with an influenza A(H1N1)pdm09 virus and occurred during week 7 (the week ending February 17, 2018). Four deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 52, 7 and 8 (the weeks ending December 30, 2017, February 17, and February 24, 2018, respectively). Nine deaths were associated with an influenza B virus and occurred during weeks 6, 7 and 8 (the weeks ending February 10, February 17, and February 24, 2018, respectively).
A total of 114 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
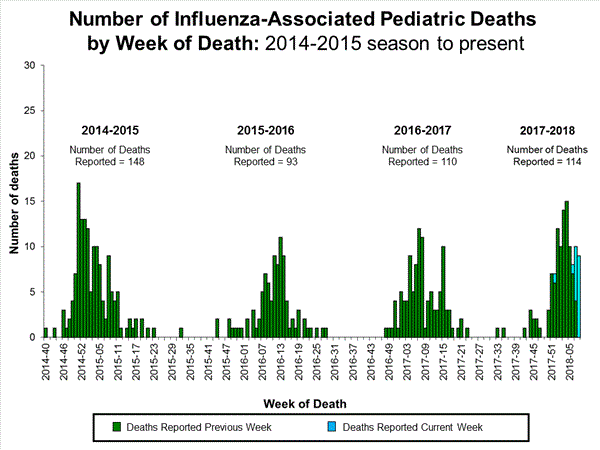
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 23,324 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and February 24, 2018. The overall hospitalization rate was 81.7 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (350.7 per 100,000 population), followed by adults aged 50-64 (88.5 per 100,000 population) and children aged 0-4 years (57.8 per 100,000 population). Among 23,324 hospitalizations, 19,026 (81.6%) were associated with influenza A virus, 4,162 (17.8%) with influenza B virus, 70 (0.3%) with influenza A virus and influenza B virus co-infection, and 66 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 4,302 (85.6%) were A(H3N2) and 721 (14.4%) were A(H1N1)pdm09 virus.
Among 2,775 hospitalized adults with information on underlying medical conditions, 1,864 (67.2%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 267 hospitalized children with information on underlying medical conditions, 118 (44.2%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 208 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 52 (25.0%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics? (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications;Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 8, 5.0% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100?F [37.8?C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.

View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation On a regional level, the percentage of outpatient visits for ILI ranged from 2.8% to 7.5% during week 8. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 8, the following ILI activity levels were experienced:- New York City, the District of Columbia, and 32 states experienced high activity (Alabama, Alaska, Arizona, Arkansas, Colorado, Georgia, Illinois, Indiana, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Vermont, Virginia, and Wyoming).
- Puerto Rico and nine states experienced moderate ILI activity (California, Connecticut, Delaware, Nevada, Ohio, Oregon, Utah, West Virginia, and Wisconsin).
- Six states experienced low ILI activity (Hawaii, Idaho, Iowa, North Dakota, Tennessee, and Washington).
- Three states experienced minimal ILI activity (Florida, Maine, and Montana).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 8, the following influenza activity was reported:- Widespread influenza activity was reported by Puerto Rico and 45 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
- Regional influenza activity was reported by Guam and two states (Minnesota and Texas).
- Local influenza activity was reported by the District of Columbia and three states (Hawaii, Oregon, and Vermont).
- No influenza activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available athttps://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
--------------------------------------------------------------------------------
File Formats Help:
How do I view different file formats (PDF, DOC, PPT, MPEG) on this site?
[/COLOR]
- Page last reviewed: March 2, 2018
Comment
-
2017-2018 Influenza Season Week 10 ending March 10, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 10 (March 4-10, 2018), influenza activity decreased in the United States.- Viral Surveillance: Overall, influenza A(H3) viruses have predominated this season. However, in recent weeks the proportion of influenza A viruses has declined, and during week 10, the numbers of influenza A and influenza B viruses reported were similar. The percentage of respiratory specimens testing positive for influenza in clinical laboratories decreased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Nine influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 89.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 3.3%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. Twelve states experienced high ILI activity; 13 states experienced moderate ILI activity; New York City and 14 states experienced low ILI activity; 11 states experienced minimal ILI activity; and Puerto Rico and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in Puerto Rico and 26 states was reported as widespread; Guam and 18 states reported regional activity; the District of Columbia and five states reported local activity; one state reported sporadic activity; and the U.S. Virgin Islands reported no activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 46 of 54 15.0% 4,415 28,950 413 722 6,562 2,748 128 Elevated 5 of 6 25.5% 249 1,723 4 43 428 38 4 Elevated 3 of 4 22.0% 263 1,628 10 15 254 279 8 Elevated 4 of 6 18.8% 858 3,153 10 159 867 93 12 Elevated 8 of 8 14.0% 779 2,705 96 32 562 531 33 Elevated 6 of 6 24.2% 692 6,349 83 48 1,149 169 24 Elevated 4 of 5 16.3% 345 1,285 8 2 355 373 22 Elevated 4 of 4 20.2% 76 1,134 12 3 434 34 2 Elevated 6 of 6 15.6% 253 2,524 17 96 714 61 2 Elevated 3 of 5 13.4% 583 7,068 166 313 1,344 769 17 Elevated 3 of 4 17.6% 317 1,381 7 11 455 401 4
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
? Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
28,157 971,395 4,223 (15.0%) 195,266 (20.1%) 1,963 (46.5%) 140,352 (71.9%) 2,260 (53.5%) 54,914 (28.1%) 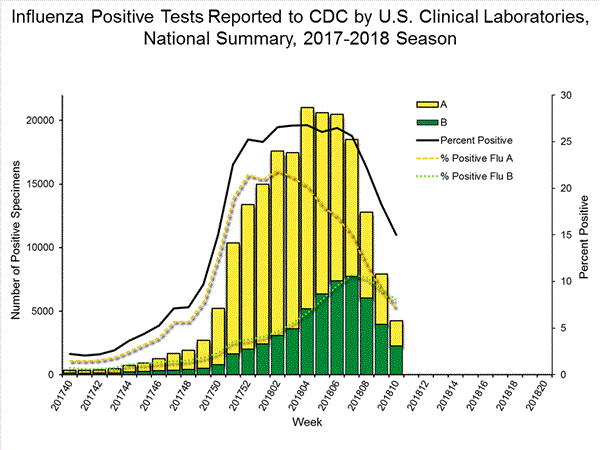
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.1,307 78,351 561 43,810 242 (43.1%) 33,778 (77.1%) 55 (22.7%) 4,415 (13.1%) 173 (71.5%) 28,950 (85.7%) 14 (5.8%) 413 (1.2%) 319 (56.9%) 10,032 (22.9%) 173 (54.2%) 6,562 (65.4%) 25 (7.8%) 722 (7.2%) 121 (37.9%) 2,748 (27.4%)
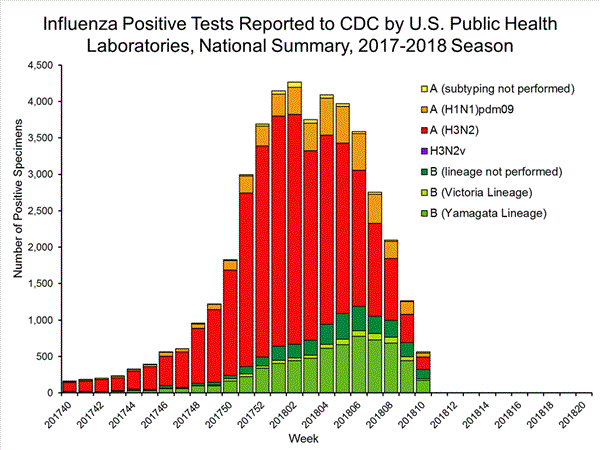
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation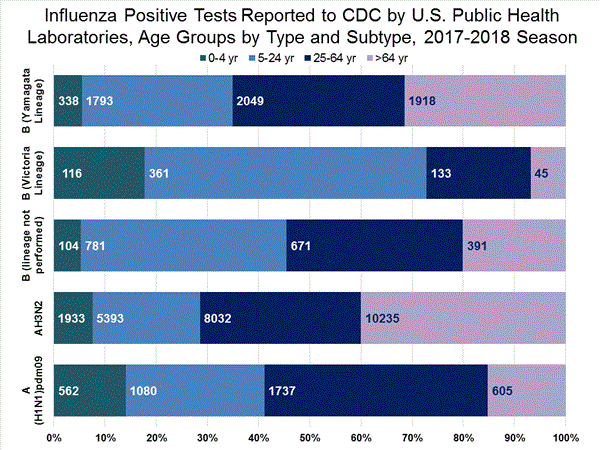
View Interactive Application | View Full Screen Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) ((i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination. On February 15, 2018, interim influenza vaccine effectiveness estimates for the 2017-2018 season were released and are available here.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 2,075 influenza viruses collected during October 1, 2017 ? March 10, 2018, and submitted by U.S. laboratories, including 499 influenza A(H1N1)pdm09 viruses, 941 influenza A(H3N2) viruses, and 635 influenza B viruses.Influenza A Viruses- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 499 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Three hundred forty three A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017?18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 941 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=792), subclade 3C.2a1 (n=127) or clade 3C.3a (n=22). Four hundred twenty nine influenza A(H3N2) viruses were antigenically characterized, and 420 (97.9%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell-propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017?18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 112 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Thirty two (43.8%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017?18 Northern Hemisphere influenza vaccines. Forty one (56.2%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 495 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 378 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell-propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017?18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmapconsiderations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
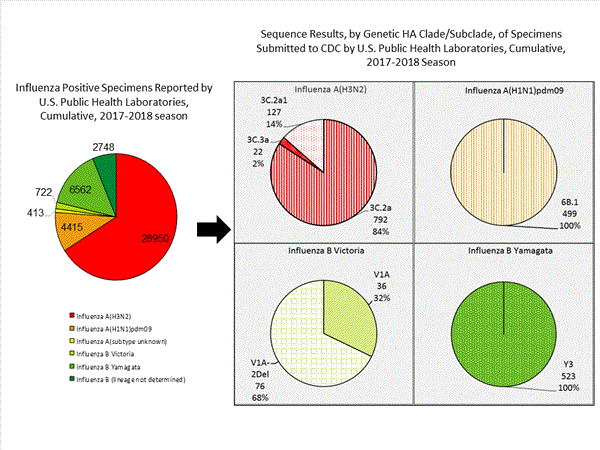
View Chart Data | View Full Screen | View PowerPoint Presentation Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.6579 (1.4)4630 (0.0)6579 (1.4)1,4530 (0.0)1,4530 (0.0)8900 (0.0)5980 (0.0)5980 (0.0)5980 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on March 15, 2018, 8.5% of the deaths occurring during the week ending February 24, 2018 (week 8) were due to P&I. This percentage is above the epidemic threshold of 7.4% for week 8.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
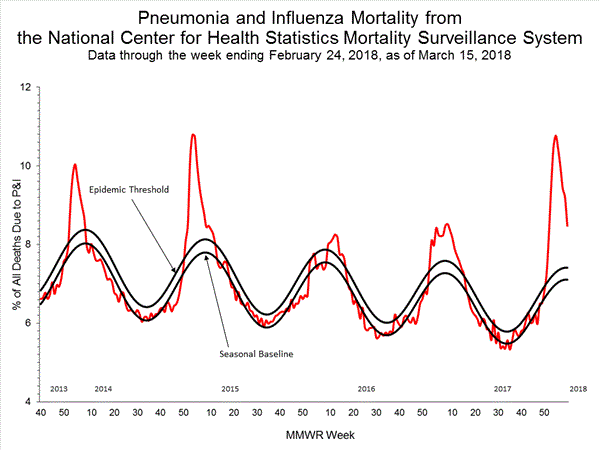
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Nine influenza-associated pediatric deaths were reported to CDC during week 10. Two deaths were associated with an influenza A(H1N1)pdm09 virus and occurred during weeks 1 and 9 (the weeks ending January 6, 2018 and March 3, 2018, respectively). Two deaths were associated with an influenza A(H3) virus and occurred during weeks 6 and 9 (the weeks ending February 10, 2018 and March 3, 2018, respectively). Five deaths were associated with an influenza B virus and occurred during weeks 6 and 9 (the weeks ending February 10, 2018, March 3, 2018, respectively).
A total of 128 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
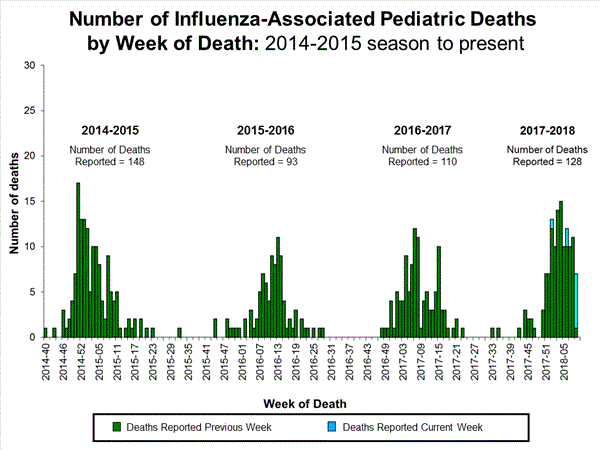
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 25,676 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and March 10, 2018. The overall hospitalization rate was 89.9 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (386.2 per 100,000 population), followed by adults aged 50-64 (97.3 per 100,000 population) and children aged 0-4 years (64.9 per 100,000 population). Among 25,676 hospitalizations, 20,293 (79.0%) were associated with influenza A virus, 5,228 (20.4%) with influenza B virus, 87 (0.3%) with influenza A virus and influenza B virus co-infection, and 68 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information 4,967 (85.2%) were A(H3N2) and 860 (14.8) were A(H1N1)pdm09 virus.
Among 2,565 hospitalized adults with information on underlying medical conditions, 2,384 (92.9%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 237 hospitalized children with information on underlying medical conditions, 130 (54.9%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 200 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 60 (30.0%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics? (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications;Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 10, 3.3% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%. (ILI is defined as fever (temperature of 100?F [37.8?C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
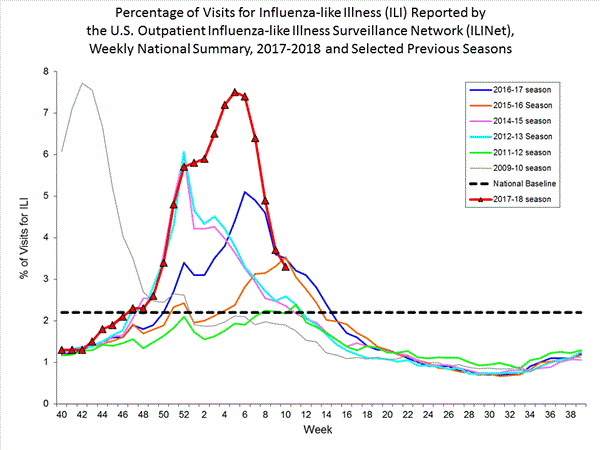
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation On a regional level, the percentage of outpatient visits for ILI ranged from 1.9% to 4.9% during week 10. All 10 regions reported percentages of outpatient visits for ILI at or above their region-specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 10, the following ILI activity levels were experienced:- Twelve states experienced high activity (Alaska, Arizona, Georgia, Kansas, Kentucky, Missouri, Nebraska, New Jersey, New Mexico, South Carolina, Virginia, and Wyoming).
- Thirteen states experienced moderate ILI activity (Arkansas, California, Hawaii, Indiana, Massachusetts, Michigan, Minnesota, New York, North Carolina, Pennsylvania, Texas, Vermont, and Wisconsin).
- New York City and 14 five states experienced low ILI activity (Alabama, Colorado, Connecticut, Illinois, Iowa, Louisiana, Maryland, Mississippi, Oklahoma, Oregon, Rhode Island, South Dakota, Utah, and West Virginia).
- Eleven states experienced minimal ILI activity (Delaware, Florida, Idaho, Maine, Montana, Nevada, New Hampshire, North Dakota, Ohio, Tennessee, and Washington).
- Data were insufficient to calculate an ILI activity level from the District of Columbia and Puerto Rico
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 10, the following influenza activity was reported:- Widespread influenza activity was reported by Puerto Rico and 26 states (Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Indiana, Kansas, Maine, Maryland, Massachusetts, Michigan, Montana, Nebraska, New Hampshire, New Jersey, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Virginia, Washington, Wisconsin, and Wyoming).
- Regional influenza activity was reported by Guam and 18 states (Alabama, Arkansas, Georgia, Idaho, Illinois, Iowa, Kentucky, Louisiana, Minnesota, Mississippi, Missouri, New Mexico, North Carolina, North Dakota, Pennsylvania, South Dakota, Tennessee, and Utah).
- Local influenza activity was reported by the District of Columbia and five states (Hawaii, Nevada, Oregon, Texas, and West Virginia).
- Sporadic influenza activity was reported by one state (Vermont).
- No influenza activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available athttps://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
--------------------------------------------------------------------------------
File Formats Help:
How do I view different file formats (PDF, DOC, PPT, MPEG) on this site?
[/COLOR]
- Page last reviewed: March 16, 2018
Comment
-
2017-2018 Influenza Season Week 11 ending March 17, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 11 (March 11-17, 2018), influenza activity decreased in the United States.- Viral Surveillance: Overall, influenza A(H3) viruses have predominated this season. However, in recent weeks the proportion of influenza A viruses has declined, and during week 11, influenza B viruses were more frequently reported than influenza A viruses. The percentage of respiratory specimens testing positive for influenza in clinical laboratories decreased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Five influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 93.5 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.7%, which is above the national baseline of 2.2%. Nine of 10 regions reported ILI at or above region-specific baseline levels. Six states experienced high ILI activity; nine states experienced moderate ILI activity; New York City, Puerto Rico, the District of Columbia, and 17 states experienced low ILI activity; and 18 states experienced minimal ILI activity.
- Geographic Spread of Influenza: The geographic spread of influenza in 17 states was reported as widespread; Guam, Puerto Rico and 26 states reported regional activity; the District of Columbia and five states reported local activity; and the U.S. Virgin Islands and two states reported sporadic activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 45 of 54 15.3% 4,609 29,524 433 916 7,208 3,016 133 Elevated 5 of 6 21.1% 257 1,745 4 60 463 38 4 Elevated 3 of 4 15.2% 271 1,725 11 32 278 312 8 Elevated 4 of 6 15.0% 904 3,291 10 174 968 94 13 Elevated 7 of 8 11.9% 794 2,755 97 32 578 557 34 Elevated 6 of 6 21.6% 714 6,452 78 53 1,265 187 24 Normal 4 of 5 11.9% 346 1,297 8 2 365 380 23 Elevated 4 of 4 18.8% 80 1,143 11 3 454 36 2 Elevated 6 of 6 15.8% 269 2,571 17 109 769 61 3 Elevated 3 of 5 14.4% 634 7,147 189 438 1,582 819 18 Elevated 3 of 4 18.4% 340 1,398 8 13 486 532 4
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
28,213 1,008,103 4,326 (15.3%) 201,060 (19.9%) 1,828 (42.3%) 142,846 (71.0%) 2,498 (57.7%) 58,214 (29.0%) 
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.896 81,677 433 45,706 184 (42.5%) 34,566 (75.6%) 67 (36.4%) 4,609 (13.3%) 100 (54.3%) 29,524 (85.4%) 17 (9.2%) 433 (1.3%) 249 (57.5%) 11,140 (24.4%) 147 (59.0%) 7,208 (64.7%) 22 (8.8%) 916 (8.2%) 80 (32.1%) 3,016 (27.1%)
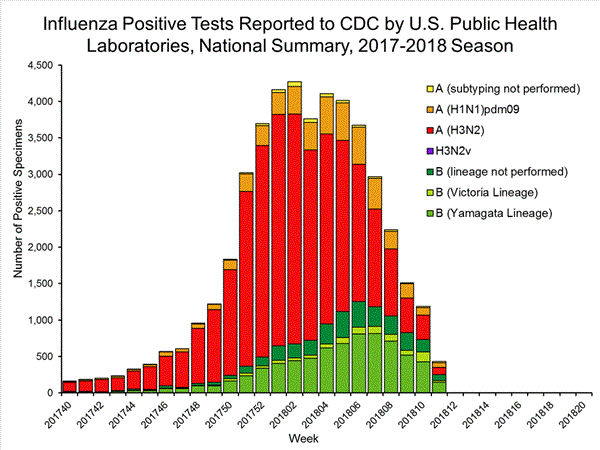
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation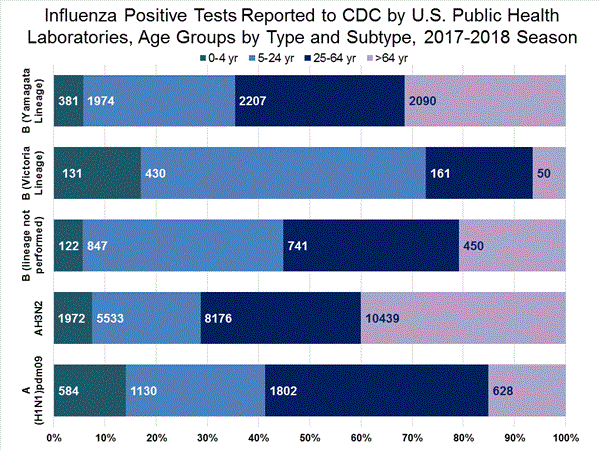
View Interactive Application | View Full Screen
Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) ((i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination. On February 15, 2018, interim influenza vaccine effectiveness estimates for the 2017-2018 season were released and are available here.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 2,214 influenza viruses collected during October 1, 2017 – March 17, 2018, and submitted by U.S. laboratories, including 535 influenza A(H1N1)pdm09 viruses, 989 influenza A(H3N2) viruses, and 690 influenza B viruses.Influenza A Viruses- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 535 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Three hundred ninety one A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017–18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 989 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=831), subclade 3C.2a1 (n=128) or clade 3C.3a (n=30). Four hundred fifty-eight influenza A(H3N2) viruses were antigenically characterized, and 446 (97.4%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell-propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017–18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 133 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Thirty-two (43.8%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell-propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017–18 Northern Hemisphere influenza vaccines. Forty-one (56.2%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 557 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 443 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell-propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017–18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmap considerations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.

View Chart Data | View Full Screen | View PowerPoint Presentation
Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available athttps://emergency.cdc.gov/han/han00409.asp.702 9 (1.3) 511 0 (0.0) 702 9 (1.3) 1,495 0 (0.0) 1,495 0 (0.0) 935 0 (0.0) 659 0 (0.0) 659 0 (0.0) 659 0 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on March 22, 2018, 7.8% of the deaths occurring during the week ending March 3, 2018 (week 9) were due to P&I. This percentage is above the epidemic threshold of 7.4% for week 9.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
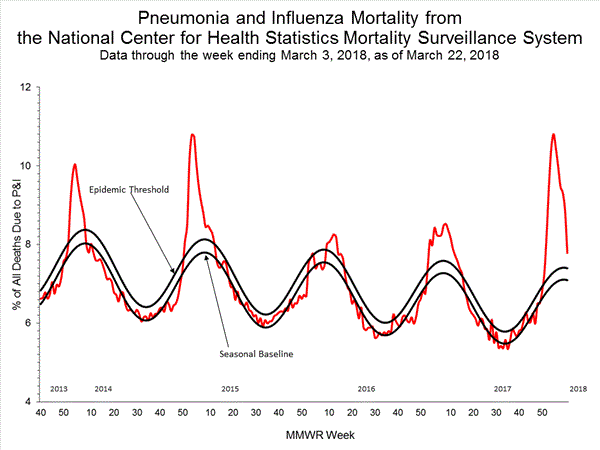
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Five influenza-associated pediatric deaths were reported to CDC during week 11. One death was associated with an influenza A virus for which subtyping was not performed and occurred during week 8 (the week ending February 24, 2018). One death was associated with an influenza A(H3) virus and occurred during week 10 (the week ending March 10, 2018). Two deaths were associated with an influenza B virus and occurred during weeks 10 and 11 (the weeks ending March 10, 2018, March 17, 2018, respectively). One death was associated with an influenza virus co-infection and occurred during week 9 (the week ending March 3, 2018).
A total of 133 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
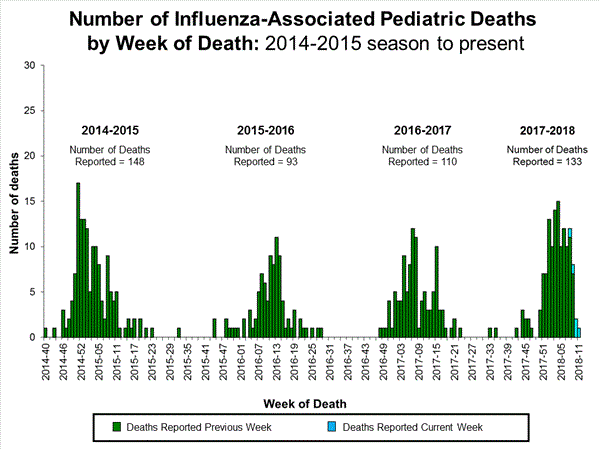
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 26,694 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and March 17, 2018. The overall hospitalization rate was 93.5 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (401.8 per 100,000 population), followed by adults aged 50-64 (101.5 per 100,000 population) and children aged 0-4 years (66.4 per 100,000 population). Among 26,694 hospitalizations, 20,731 (77.7%) were associated with influenza A virus, 5,803 (21.7%) with influenza B virus, 92 (0.3%) with influenza A virus and influenza B virus co-infection, and 68 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 5,187 (84.9%) were A(H3N2) and 922 (15.1%) were A(H1N1)pdm09 virus.
Among 2,830 hospitalized adults with information on underlying medical conditions, 2,630 (92.9%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 253 hospitalized children with information on underlying medical conditions, 138 (54.5%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 214 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 63 (29.4%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics’ (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation
FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppressionincludes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications; Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 11, 2.7% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%. (ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
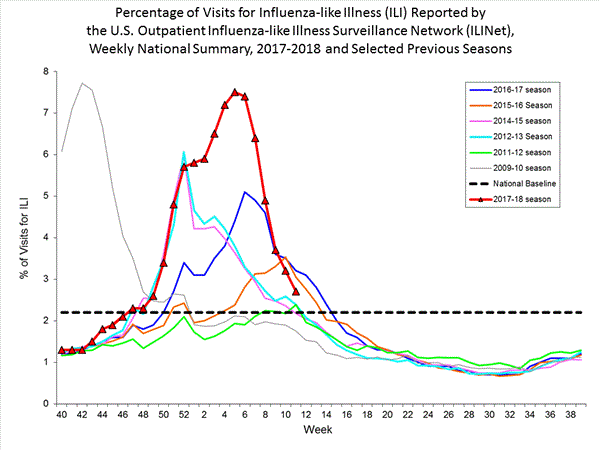
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 1.8% to 3.4% during week 11. Nine of ten 10 regions (Regions 1, 2, 3, 4, 5, 7, 8, 9, and 10) reported percentages of outpatient visits for ILI at or above their region-specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 11, the following ILI activity levels were experienced:- Six states experienced high activity (Arizona, Nebraska, South Carolina, South Dakota, Virginia, and Wyoming).
- Nine states experienced moderate ILI activity (California, Georgia, Kansas, Massachusetts, New Jersey, New Mexico, North Carolina, Pennsylvania, and Rhode Island).
- New York City, Puerto Rico, the District of Columbia, and 17 five states experienced low ILI activity (Alabama, Alaska, Arkansas, Colorado, Hawaii, Illinois, Indiana, Iowa, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Nevada, Oregon, Vermont, and Washington).
- Eighteen states experienced minimal ILI activity (Connecticut, Delaware, Florida, Idaho, Maine, Maryland, Missouri, Montana, New Hampshire, New York, North Dakota, Ohio, Oklahoma, Tennessee, Texas, Utah, West Virginia, and Wisconsin).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 11, the following influenza activity was reported:- Widespread influenza activity was reported by 17 states (Alaska, California, Connecticut, Delaware, Indiana, Maine, Maryland, Massachusetts, Nebraska, New Hampshire, New Jersey, New York, Ohio, Oklahoma, Rhode Island, Virginia, and Wisconsin).
- Regional influenza activity was reported by Guam, Puerto Rico and 26 states (Alabama, Arizona, Arkansas, Colorado, Florida, Georgia, Idaho, Illinois, Iowa, Kansas, Kentucky, Michigan, Minnesota, Mississippi, Missouri, Montana, New Mexico, North Carolina, North Dakota, Pennsylvania, South Carolina, South Dakota, Texas, Utah, Washington, and Wyoming).
- Local influenza activity was reported by the District of Columbia and five states (Louisiana, Nevada, Oregon, Tennessee, and West Virginia).
- Sporadic influenza activity was reported by the U.S. Virgin Islands and two states (Hawaii and Vermont).
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available at https://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
Comment
-
2017-2018 Influenza Season Week 12 ending March 24, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 12 (March 18-24, 2018), influenza activity decreased in the United States.- Viral Surveillance: Overall, influenza A(H3) viruses have predominated this season. However, in recent weeks the proportion of influenza A viruses has declined, and during week 12, influenza B viruses were more frequently reported than influenza A viruses. The percentage of respiratory specimens testing positive for influenza in clinical laboratories decreased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Four influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 96.1 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.5%, which is above the national baseline of 2.2%. Nine of 10 regions reported ILI at or above region-specific baseline levels. Four states experienced high ILI activity; eight states experienced moderate ILI activity; New York City, Puerto Rico, the District of Columbia, and 14 states experienced low ILI activity; and 24 states experienced minimal ILI activity.
- Geographic Spread of Influenza: The geographic spread of influenza in Puerto Rico and 16 states was reported as widespread; 22 states reported regional activity; the District of Columbia, Guam and eight states reported local activity; four states reported sporadic activity; and the U.S. Virgin Islands reported no influenza activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 39 of 54 14.7% 4,796 30,048 571 890 7,697 3,174 137 Elevated 5 of 6 17.6% 265 1,766 5 62 465 44 4 Elevated 3 of 4 12.4% 281 1,758 21 34 330 292 8 Elevated 4 of 6 14.5% 937 3,406 12 187 1,079 99 13 Elevated 4 of 8 11.3% 818 2,789 97 32 603 595 35 Elevated 6 of 6 21.3% 733 6,501 81 56 1,410 196 24 Normal 2 of 5 9.2% 351 1,304 8 2 378 400 25 Elevated 4 of 4 15.3% 80 1,153 9 4 464 35 2 Elevated 6 of 6 16.1% 275 2,598 22 113 800 70 4 Elevated 2 of 5 12.7% 704 7,367 308 387 1,677 891 18 Elevated 3 of 4 19.2% 352 1,406 8 13 491 552 4
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
24,329 1,045,697 3,575 (14.7%) 207,104 (19.8%) 1,509 (42.2%) 145,494 (70.3%) 2,066 (57.8%) 61,610 (29.7%) 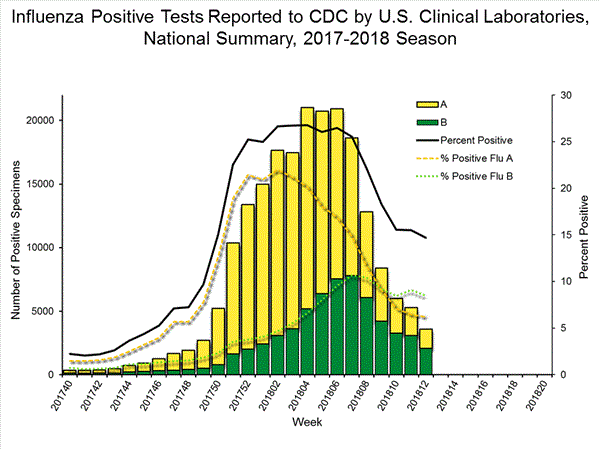
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.935 84,437 368 47,176 131 (35.6%) 35,415 (75.1%) 34 (26.0%) 4,796 (13.5%) 90 (68.7%) 30,048 (84.8%) 7 (5.3%) 571 (1.6%) 237 (64.4%) 11,761 (24.9%) 149 (62.9%) 7,697 (65.4%) 11 (4.6%) 890 (7.6%) 77 (32.5%) 3,174 (27.0%)
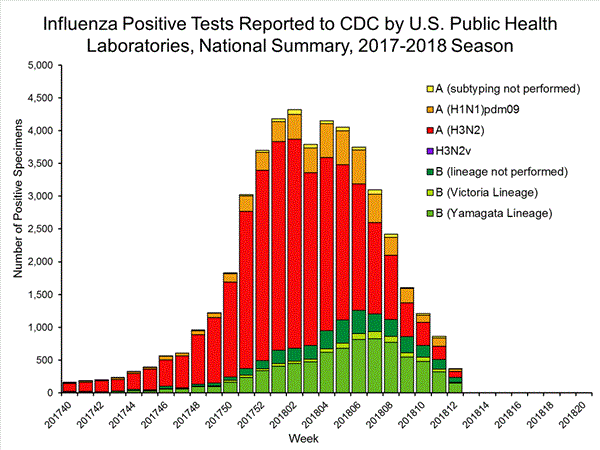
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation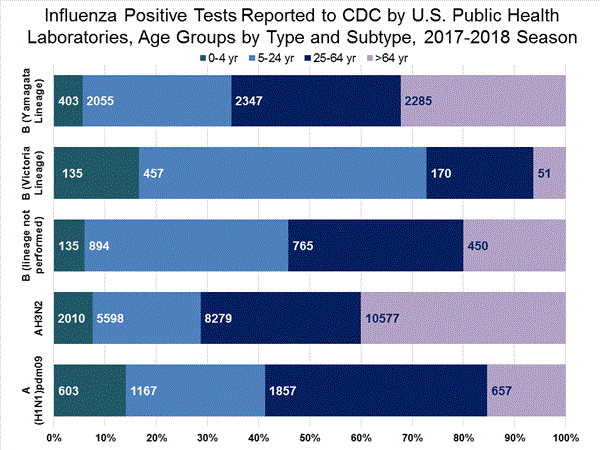
View Interactive Application | View Full Screen
Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) ((i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination. On February 15, 2018, interim influenza vaccine effectiveness estimates for the 2017-2018 season were released and are available here.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 2,384 influenza viruses collected during October 1, 2017 – March 24, 2018, and submitted by U.S. laboratories, including 578 influenza A(H1N1)pdm09 viruses, 1,042 influenza A(H3N2) viruses, and 764 influenza B viruses.Influenza A Viruses- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 578 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Four hundred fifty-three A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017–18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 1,042 A(H3N2) viruses revealed extensive genetic diversity with? multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=873), subclade 3C.2a1 (n=131) or clade 3C.3a (n=38). Four hundred sixty-nine influenza A(H3N2) viruses were antigenically characterized, and 456 (97.2%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell-propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of? 2017–18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 153 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Thirty four (28.6%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell-propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017–18 Northern Hemisphere influenza vaccines. Eighty-five (71.4%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 611 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 443 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell-propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017–18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmap considerations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
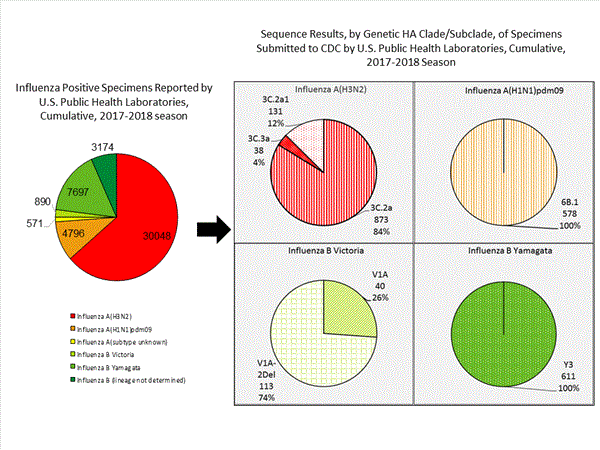
View Chart Data | View Full Screen | View PowerPoint Presentation
2018-2019 Influenza Season – U.S. Influenza Vaccine Composition:
The World Health Organization (WHO) has recommended the Northern Hemisphere 2018-2019 influenza vaccine composition, and the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) subsequently made the influenza vaccine composition recommendation for the United States. Both agencies recommend that influenza trivalent vaccines contain an A/Michigan/45/2015 (H1N1)pdm09-like virus, an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and a B/Colorado/06/2017-like (B/Victoria lineage) virus. It is recommended that quadrivalent vaccines, which have two influenza B viruses, contain the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like (B/Yamagata lineage) virus. The B recommendation represents a change in the influenza B/Victoria lineage component recommended for 2017-2018 Northern Hemisphere and 2018 Southern Hemisphere influenza vaccines. The H3N2 recommendation represents an update to the 2017-2018 Northern Hemisphere vaccines, but not to the 2019 Southern Hemisphere vaccines which were recommended to include A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus. The B component change was made because of the detection and increasing global circulation of an antigenically drifted B/Victoria lineage virus. The update to the H3N2 component is not a result of antigenic drift, but because the egg-propagated A/Singapore vaccine virus is antigenically more similar to circulating viruses than the egg-propageted A/Hong Kong vaccine virus recommended for the Northern Hemisphere 2017-2018 vaccines. These vaccine recommendations were based on several factors, including global influenza virologic and epidemiologic surveillance, genetic characterization, antigenic characterization and the candidate vaccine viruses that are available for production.
Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available athttps://emergency.cdc.gov/han/han00409.asp.770 9 (1.2) 532 0 (0.0) 770 9 (1.2) 1,671 0 (0.0) 1,671 0 (0.0) 972 0 (0.0) 696 0 (0.0) 696 0 (0.0) 696 0 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on March 29, 2018, 7.7% of the deaths occurring during the week ending March 10, 2018 (week 10) were due to P&I. This percentage is above the epidemic threshold of 7.4% for week 10.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
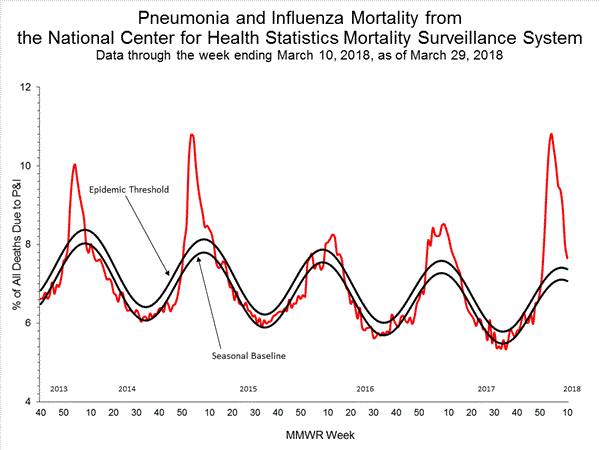
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Four influenza-associated pediatric deaths were reported to CDC during week 12. ? One death was associated with an influenza A(H3) virus and occurred during week 51 (the week ending December 23, 2017).? Two deaths were associated with an influenza A virus for which subtyping was not performed and occurred during weeks 8 and 11 (the week ending February 24 and March 17, 2018, respectively).? One death was associated with an influenza B virus and occurred during week 6 (the week ending February 10, 2018, respectively).
A total of 137 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.

View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 27,438 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and March 24, 2018. The overall hospitalization rate was 96.1 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (412.6 per 100,000 population), followed by adults aged 50-64 (104.2 per 100,000 population) and children aged 0-4 years (68.7 per 100,000 population). Among 27,438 hospitalizations, 21,014 (76.6%) were associated with influenza A virus, 6,253 (22.8%) with influenza B virus, 95 (0.3%) with influenza A virus and influenza B virus co-infection, and 76 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 5,373 (85.2%) were A(H3N2) and 935 (14.8) were A(H1N1)pdm09 virus.
Among 3,102 hospitalized adults with information on underlying medical conditions, 2,884 (93.0%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 277 hospitalized children with information on underlying medical conditions, 152 (54.9%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 232 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 70 (30.2%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics’ (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation
FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppressionincludes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications; Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 12, 2.5% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%. (ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
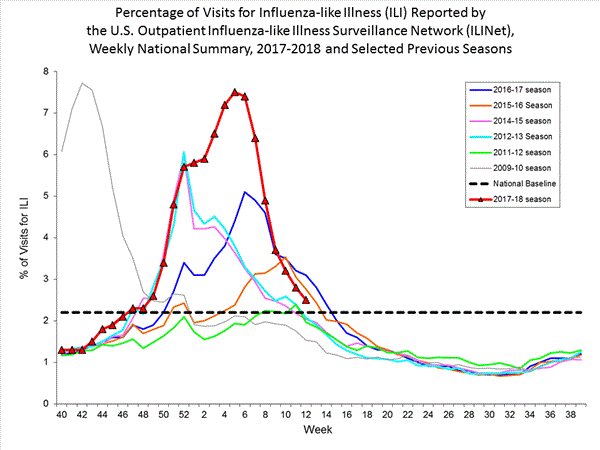
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 1.3% to 3.3% during week 12. Nine of ten 10 regions (Regions 1, 2, 3, 4, 5, 7, 8, 9, and 10) reported percentages of outpatient visits for ILI at or above their region-specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 12, the following ILI activity levels were experienced:- Four states experienced high activity (Alaska, South Carolina, Virginia, and Wyoming).
- Eight states experienced moderate ILI activity (Arizona, Indiana, Kentucky, Missouri, Nebraska, New Mexico, Pennsylvania, and South Dakota).
- New York City, Puerto Rico, the District of Columbia, and 14 states experienced low ILI activity (California, Connecticut, Georgia, Hawaii, Illinois, Kansas, Massachusetts, Minnesota, New Jersey, North Carolina, Oregon, Rhode Island, Vermont, and Washington).
- Twenty-four states experienced minimal ILI activity (Alabama, Arkansas, Colorado, Delaware, Florida, Idaho, Iowa, Louisiana, Maine, Maryland, Michigan, Mississippi, Montana, Nevada, New Hampshire, New York, North Dakota, Ohio, Oklahoma, Tennessee, Texas, Utah, West Virginia, and Wisconsin).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 12, the following influenza activity was reported:- Widespread influenza activity was reported by Puerto Rico and 16 states (Alaska, California, Connecticut, Delaware, Indiana, Maine, Maryland, Massachusetts, Nebraska, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, Virginia, and Wisconsin).
- Regional influenza activity was reported by 22 states (Arizona, Colorado, Florida, Georgia, Idaho, Illinois, Iowa, Kansas, Kentucky, Michigan, Minnesota, Missouri, Montana, New Jersey, New Mexico, North Dakota, Pennsylvania, South Carolina, South Dakota, Utah, Washington, and Wyoming).
- Local influenza activity was reported by the District of Columbia, Guam and eight states (Arkansas, Louisiana, Nevada, North Carolina, Oregon, Tennessee, Texas, and West Virginia).
- Sporadic influenza activity was reported by four states (Alabama, Hawaii, Mississippi, and Vermont).
- No influenza activity was reported by the U.S. Virgin Islands.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available at https://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
Comment
-
2017-2018 Influenza Season Week 13 ending March 31, 2018
All data are preliminary and may change as more reports are received.
Synopsis:
During week 13 (March 25-31, 2018), influenza activity decreased in the United States.- Viral Surveillance: Overall, influenza A(H3) viruses have predominated this season. Since early March, influenza B viruses have been more frequently reported than influenza A viruses. The percentage of respiratory specimens testing positive for influenza in clinical laboratories remains elevated.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Five influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 99.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.4%, which is above the national baseline of 2.2%. Seven of 10 regions reported ILI at or above region-specific baseline levels. Two states experienced high ILI activity; eight states experienced moderate ILI activity; New York City and 12 states experienced low ILI activity; and the District of Columbia, Puerto Rico, and 28 states experienced minimal ILI activity.
- Geographic Spread of Influenza: The geographic spread of influenza in 11 states was reported as widespread; Guam, Puerto Rico and 26 states reported regional activity; the District of Columbia and 10 states reported local activity; and the U.S. Virgin Islands and three states reported sporadic activity.
*https://www.hhs.gov/about/agencies/i...ces/index.htmlElevated 39 of 54 15.4% 4,976 30,378 580 974 8,297 3,139 142 Elevated 6 of 6 16.5% 274 1,777 5 67 499 47 4 Normal 3 of 4 13.8% 284 1,770 22 34 342 305 8 Elevated 4 of 6 15.4% 999 3,559 10 217 1,224 105 13 Elevated 4 of 8 11.4% 835 2,815 97 36 660 593 36 Elevated 5 of 6 22.8% 756 6,534 82 61 1,476 221 27 Normal 1 of 5 7.3% 352 1,308 8 4 497 296 25 Elevated 4 of 4 12.4% 82 1,162 9 5 485 36 3 Normal 6 of 6 14.2% 294 2,623 23 141 859 73 4 Elevated 3 of 5 10.9% 723 7,410 312 396 1,742 873 18 Elevated 3 of 4 18.8% 377 1,420 12 13 513 590 4
? Elevated means the % of visits for ILI is at or above the national or region-specific baseline
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
? National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/flu...dashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
21,823 1,073,126 3,357 (15.4%) 211,428 (19.7%) 1,329 (39.6%) 147,096 (69.6%) 2,028 (60.4%) 64,332 (30.4%) 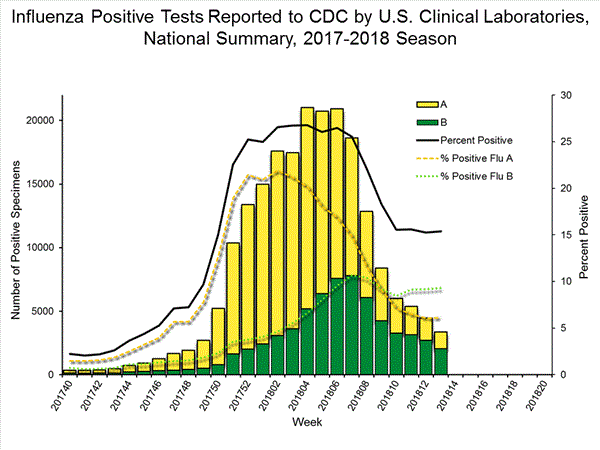
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.775 86,725 313 48,344 120 (38.3%) 35,934 (74.3%) 41 (34.2%) 4,976 (13.8%) 73 (60.8%) 30,378 (84.5%) 6 (5.0%) 580 (1.6%) 193 (61.7%) 12,410 (25.7%) 113 (58.5%) 8,297 (66.9%) 19 (9.8%) 974 (7.8%) 61 (31.6%) 3,139 (25.3%)
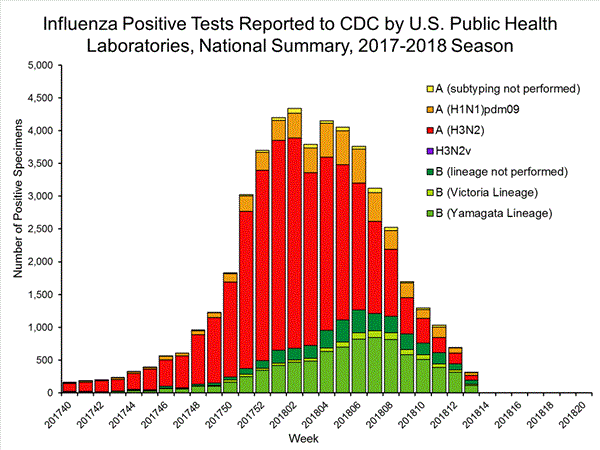
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation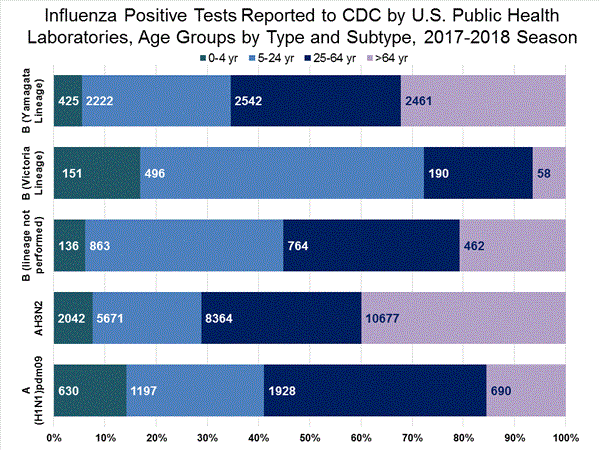
View Interactive Application | View Full Screen
Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) (i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination. On February 15, 2018, interim influenza vaccine effectiveness estimates for the 2017-2018 season were released and are available here.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
CDC has antigenically or genetically characterized 2,422 influenza viruses collected during October 1, 2017 – March 31, 2018, and submitted by U.S. laboratories, including 589 influenza A(H1N1)pdm09 viruses, 1,053 influenza A(H3N2) viruses, and 780 influenza B viruses.Influenza A Viruses- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 589 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Four hundred ninety-seven A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017–18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 1,053 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=883), subclade 3C.2a1 (n=131) or clade 3C.3a (n=39). Five hundred eleven influenza A(H3N2) viruses were antigenically characterized, and 495 (96.9%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell-propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of the 2017–18 Northern Hemisphere influenza vaccines.
- B/Victoria: Phylogenetic analysis of 158 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Thirty-seven (28.0%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell-propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of the 2017–18 Northern Hemisphere influenza vaccines. Ninety-five (72.0%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 622 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 475 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017–18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmap considerations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
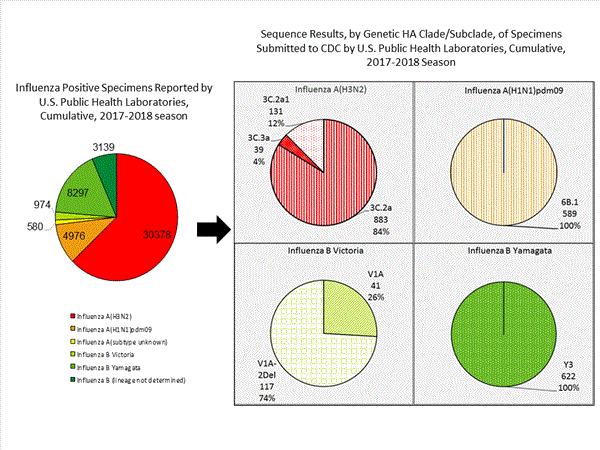
View Chart Data | View Full Screen | View PowerPoint Presentation
2018-2019 Influenza Season – U.S. Influenza Vaccine Composition:
The World Health Organization (WHO) has recommended the Northern Hemisphere 2018-2019 influenza vaccine composition, and the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) subsequently made the influenza vaccine composition recommendation for the United States. Both agencies recommend that influenza trivalent vaccines contain an A/Michigan/45/2015 (H1N1)pdm09-like virus, an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and a B/Colorado/06/2017-like (B/Victoria lineage) virus. It is recommended that quadrivalent vaccines, which have two influenza B viruses, contain the viruses recommended for the trivalent vaccines, as well as a B/Phuket/3073/2013-like (B/Yamagata lineage) virus. The B recommendation represents a change in the influenza B/Victoria lineage component recommended for the 2017-2018 Northern Hemisphere and 2018 Southern Hemisphere influenza vaccines. The H3N2 recommendation represents an update to the 2017-2018 Northern Hemisphere vaccines, but not to the 2018 Southern Hemisphere vaccines which were recommended to include A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus. The B component change was made because of the detection and increasing global circulation of an antigenically drifted B/Victoria lineage virus. The update to the H3N2 component is not a result of antigenic drift, but because the egg-propagated A/Singapore vaccine virus is antigenically more similar to circulating viruses than the egg-propagated A/Hong Kong vaccine virus recommended for the Northern Hemisphere 2017-2018 vaccines. These vaccine recommendations were based on several factors, including global influenza virologic and epidemiologic surveillance, genetic characterization, antigenic characterization and the candidate vaccine viruses that are available for production.
Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available athttps://emergency.cdc.gov/han/han00409.asp.841 9 (1.1) 568 0 (0.0) 841 9 (1.1) 1,739 0 (0.0) 1,739 0 (0.0) 1,009 0 (0.0) 752 0 (0.0) 752 0 (0.0) 752 0 (0.0)
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on April 5, 2018, 7.1% of the deaths occurring during the week ending March 17, 2018 (week 11) were due to P&I. This percentage is below the epidemic threshold of 7.3% for week 11.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. There is currently a delay in manual coding for deaths occurring in 2018. Because of this delay initially reported P&I percentages will be lower than those calculated from the final data.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
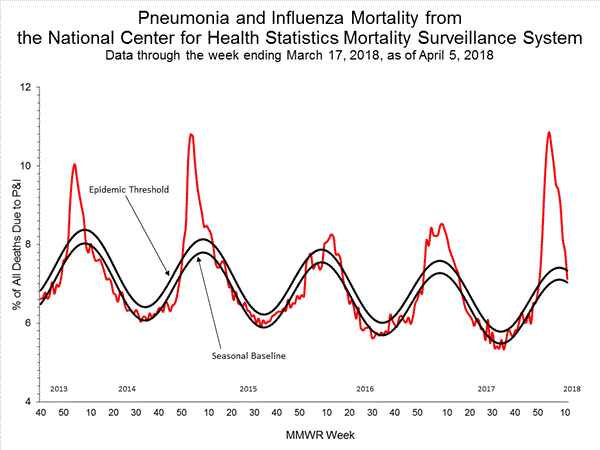
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
Five influenza-associated pediatric deaths were reported to CDC during week 13. ? One death was associated with an influenza A(H3) virus and occurred during week 13 (the week ending March 31, 2018).? Two deaths were associated with an influenza A virus for which subtyping was not performed and occurred during weeks 9 and 12 (the week ending March 3 and March 24, 2018, respectively).? Two deaths were associated with an influenza B virus and occurred during weeks 12 and 13 (the weeks ending March 24 and March 31, 2018, respectively).
A total of 142 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
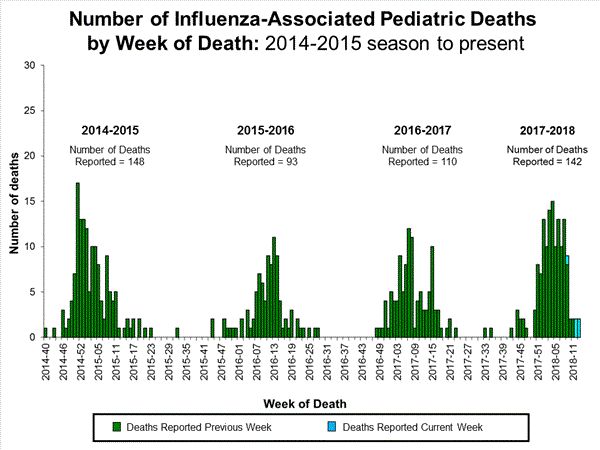
View Interactive Application | View Full Screen | View PowerPoint Presentation
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 28,543 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and March 31, 2018. The overall hospitalization rate was 99.9 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (429.4 per 100,000 population), followed by adults aged 50-64 (108.7 per 100,000 population) and children aged 0-4 years (71.2 per 100,000 population). Among 28,543 hospitalizations, 21,530 (75.4%) were associated with influenza A virus, 6,835 (23.9%) with influenza B virus, 101 (0.4%) with influenza A virus and influenza B virus co-infection, and 77 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 5,456 (85.0%) were A(H3N2) and 961 (15.0) were A(H1N1)pdm09 virus.
Among 3,446 hospitalized adults with information on underlying medical conditions, 3,206 (93.0%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 309 hospitalized children with information on underlying medical conditions, 169 (54.7%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 253 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 77 (30.4%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics’ (NCHS) population estimates for the counties included in the surveillance catchment area.
View Interactive Application | View Full Screen | View PowerPoint Presentation
FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppressionincludes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications; Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient's medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
View Interactive Application | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance:
Nationwide during week 13, 2.4% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%. (ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/flu...dashboard.html.
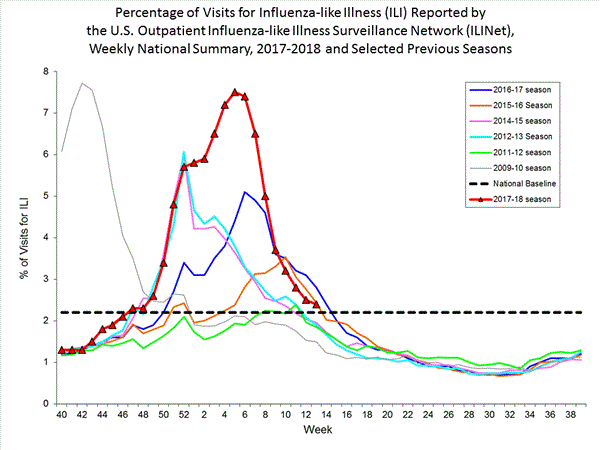
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 1.1% to 2.9% during week 13. Seven of ten 10 regions (Regions 1, 3, 4, 5, 7, 9, and 10) reported percentages of outpatient visits for ILI at or above their region-specific baselines.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 13, the following ILI activity levels were experienced:- Two states experienced high ILI activity (Alaska and Virginia).
- Eight states experienced moderate ILI activity (Arizona, Kentucky, Michigan, Nebraska, North Carolina, South Carolina, South Dakota, and Wyoming).
- New York City and 12 states experienced low ILI activity (California, Georgia, Indiana, Kansas, Massachusetts, Minnesota, New Jersey, New Mexico, Oregon, Pennsylvania, Vermont, and West Virginia).
- The District of Columbia, Puerto Rico, and 28 states experienced minimal ILI activity (Alabama, Arkansas, Colorado, Connecticut, Delaware, Florida, Hawaii, Idaho, Illinois, Iowa, Louisiana, Maine, Maryland, Mississippi, Missouri, Montana, Nevada, New Hampshire, New York, North Dakota, Ohio, Oklahoma, Rhode Island, Tennessee, Texas, Utah, Washington, and Wisconsin).
Click on map to launch interactive tool*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
Additional data can be found at https://gis.cdc.gov/grasp/fluview/FluView8.html.
During week 13, the following influenza activity was reported:- Widespread influenza activity was reported by 11 states (California, Connecticut, Delaware, Maryland, Massachusetts, New Hampshire, New York, Ohio, Rhode Island, Virginia, and Wisconsin).
- Regional influenza activity was reported by Guam, Puerto Rico and 26 states (Alaska, Arizona, Colorado, Florida, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Maine, Michigan, Missouri, Montana, Nebraska, New Jersey, North Carolina, North Dakota, Oklahoma, Pennsylvania, South Carolina, South Dakota, Utah, Vermont, Washington, and Wyoming).
- Local influenza activity was reported by the District of Columbia and 10 states (Arkansas, Georgia, Hawaii, Louisiana, Minnesota, Nevada, New Mexico, Oregon, Texas, and West Virginia).
- Sporadic influenza activity was reported by the U.S. Virgin Islands and three states (Alabama, Tennessee, and Mississippi).
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visithttp://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available at https://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
Comment
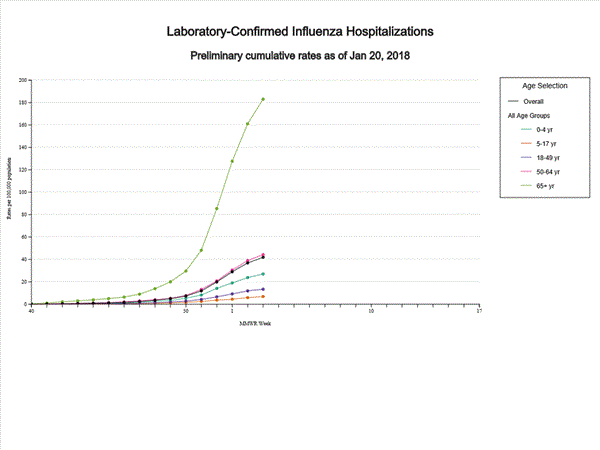
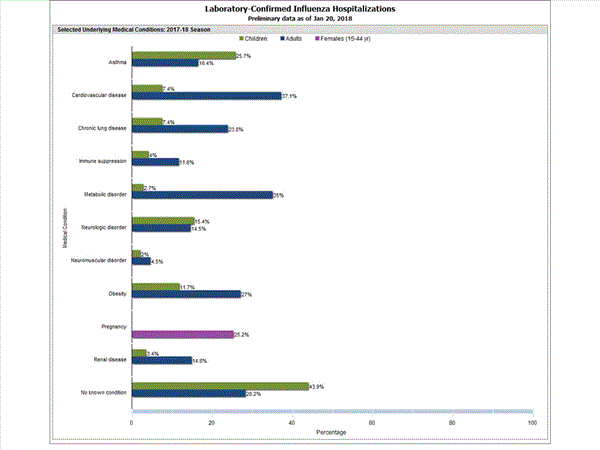
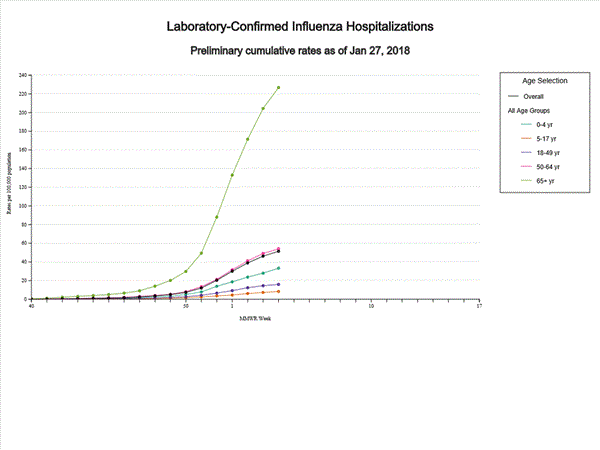
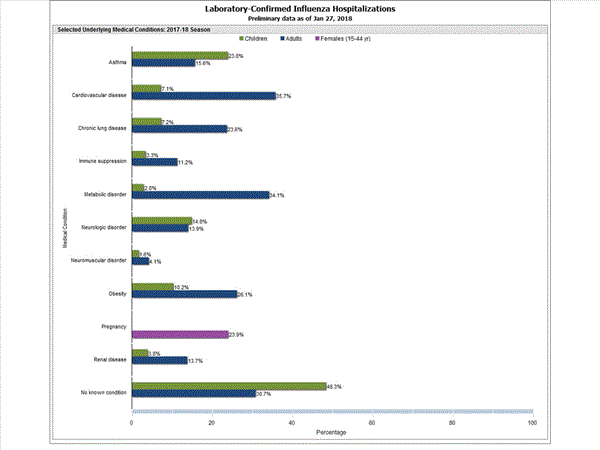
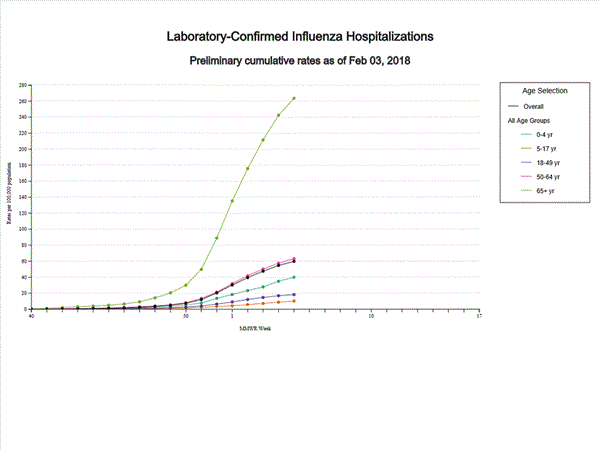
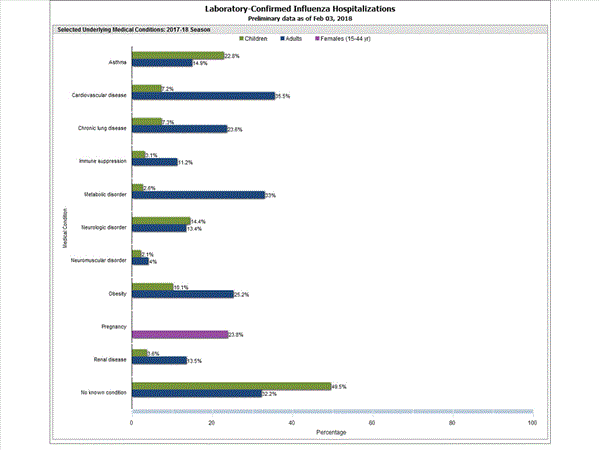

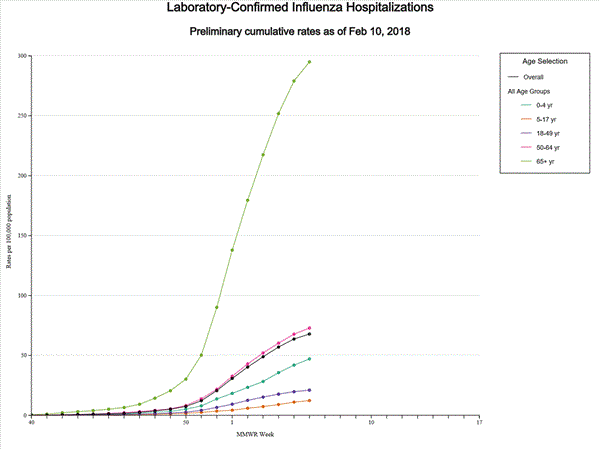
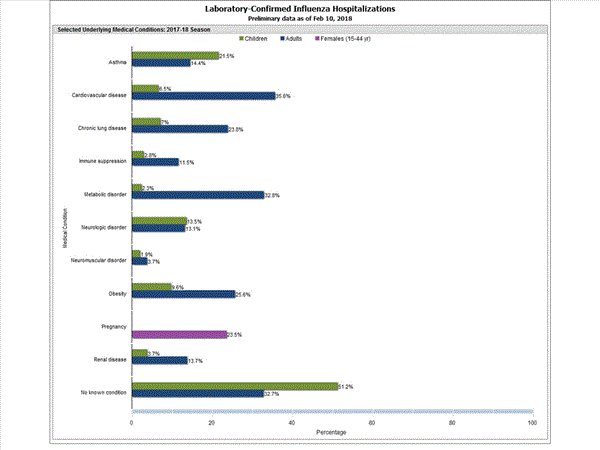
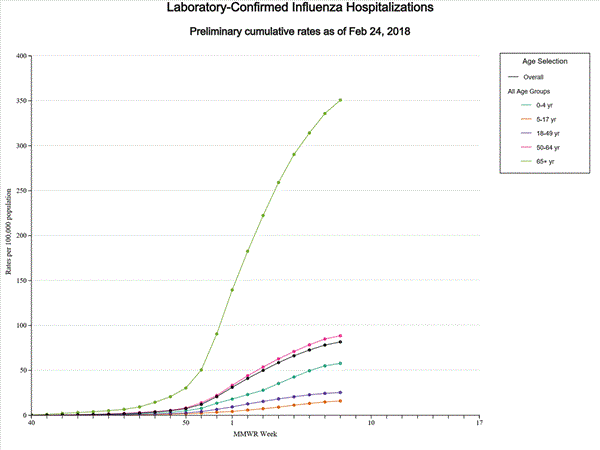
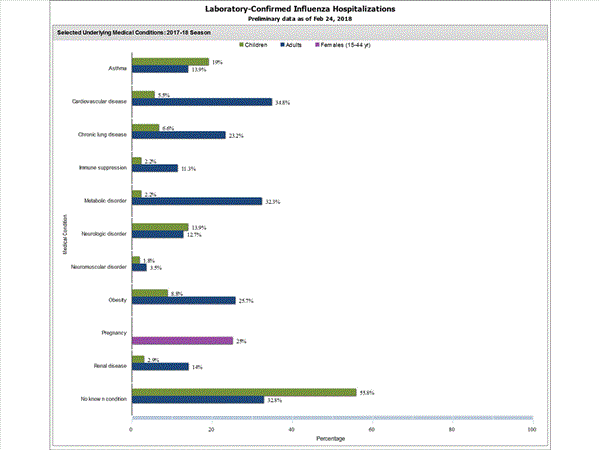
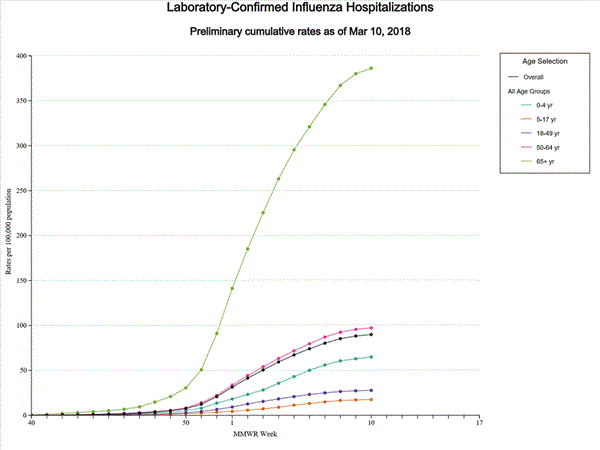
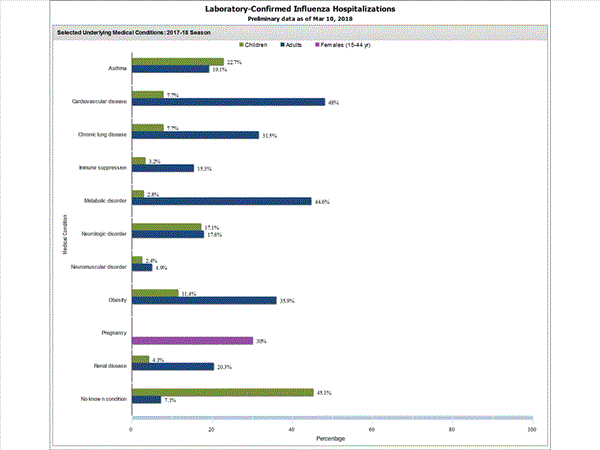
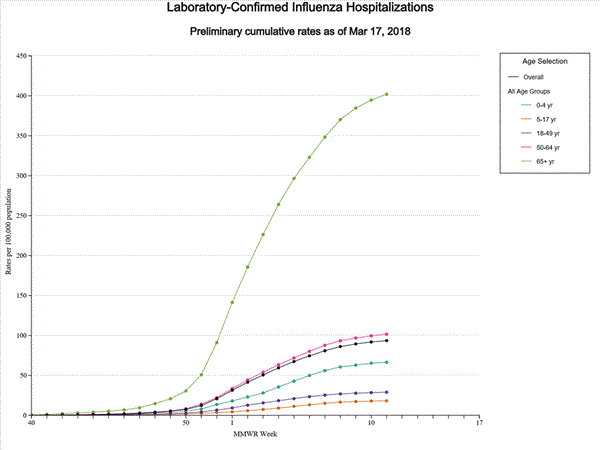
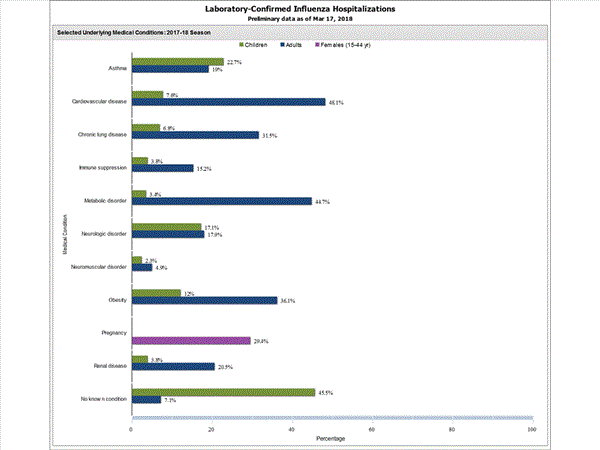
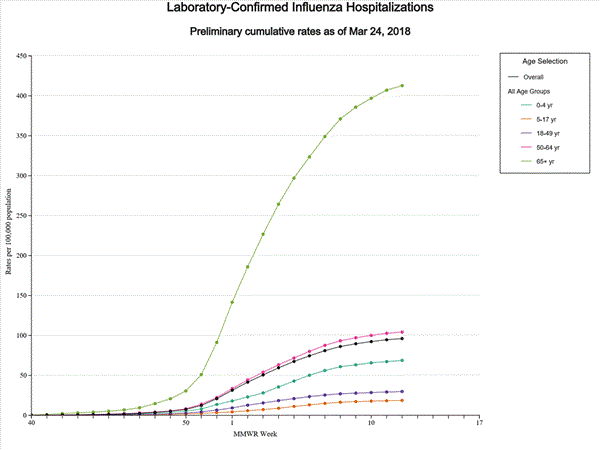
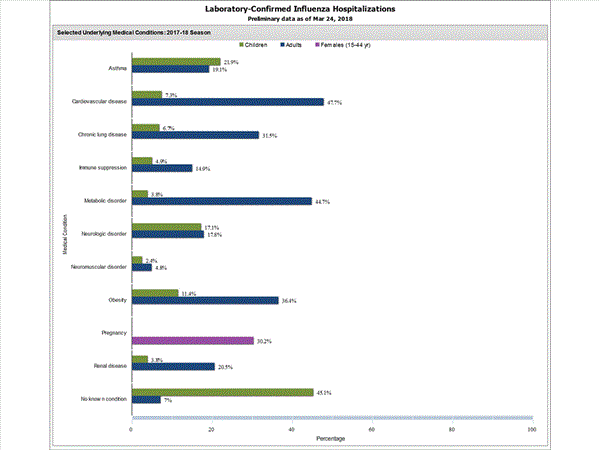
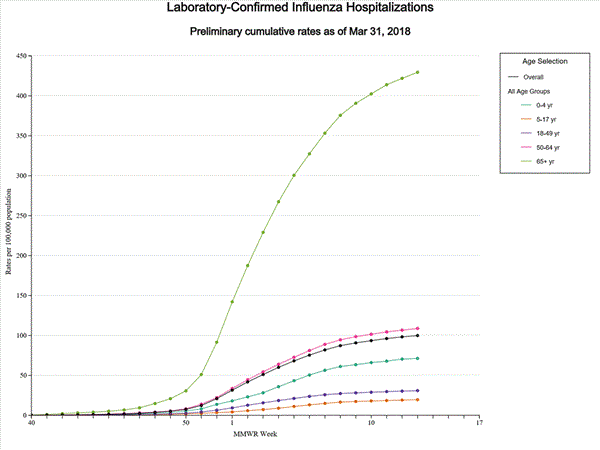
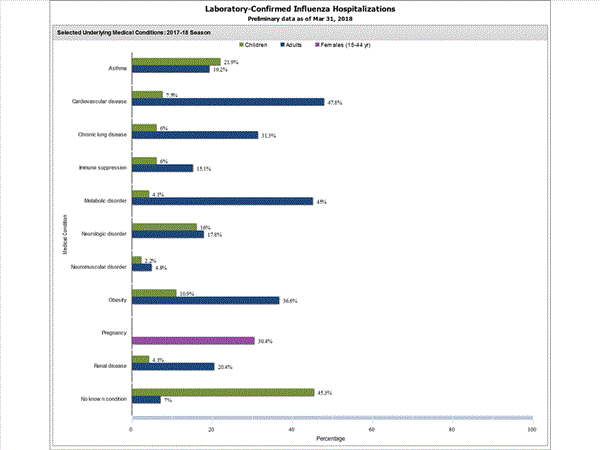
Comment