This post is just to provide two links to episodes of Immune which are excellent and helpful in understanding B cells and their maturation.
If you are not very familiar with this topic it may be helpful to read post #14 in this thread first.
Immune 52: B cell boot camp with Gabriel Victora
Immune 74: Germinal center dynamics with Carla Nowosad
Announcement
Collapse
No announcement yet.
COVID-19 , immunity
Collapse
X
-
There is a lot being written about the Omicron variant attempting to explain how it is impacting immunity and, in the interest of keeping it simple and understandable, much is being lost.
The aim of this post is to look at the interaction of the virus and host’s immune system to try and reach a more nuanced understanding of what is likely to be going on.
Natural infection will produce a very broad range of B and T cell responses but in a rather scatter gun manner. The vaccines, with the exception of one from China, only target the spike protein which concentrates all its effort on the most useful antigenic sites capable of making neutralising antibodies which can block infection. This comes at the expense of ignoring 90% of the genome. The vaccines were developed to limit severity and deaths in the hope that at least one of them would be able to do so by at least 50%. They have wildly exceeded expectations with several exceeding 70% and some into the 90s. As an ancillary benefit they happen to have also cut infections by about 50%, which was not by design and was not anticipated. They are given intramuscularly, which is good for IgG production, these circulate in the blood which gets them all over the body. The primary site of infection in the nasal cavity where, dimeric IgA is the primary source of infection control, and these will be produced after the infection starts - which is why the vaccines are better at stopping severe disease than infection. The initial infection then spreads into the lungs which is where the damage is done requiring oxygen support and hospitalisation. Any reduction of viral load at infection, or slowing of subsequent spread, buys time for the immune response to ramp up and clear the virus.
In Omicron’s case there are 36 changes in the spike protein sequence which will inevitably change the structure of the spike trimer. The antibodies, matched to the earlier variants, will bind more weakly and neutralisation assays to sera from previously infected or vaccinated hosts are showing this. Early data is also showing that the combination of prior infection with vaccination is giving better neutralisation than two vaccine doses. There are two probable reasons for this firstly those vaccinated do not have the backup of antibodies, or T cells, to all the non-spike proteins. Secondly the vaccine will only produce antibodies to the spike antigenic sites found on the protein included in the vaccine. In a natural infection the virus operates as a quasi-species, with many different variants produced in the course of the infection, and the immune response will be to all of these. This increases the chance that at least some of the B and T cells will start off as better matches to Omicron’s structure. Regardless of how good the initial match is the immune system will start amending its immune cell mix to match the current infection by the process of hyper mutation in the germinal centres (which I looked at in an earlier post in this thread). Again it is all to do with how much whatever you start with can buy time for the immune system to get a well matched response. Patients who have a condition that stops them making B cells, which make the IgG antibodies, do surprisingly well.
The next thing to consider are the tests and correlates of protection. A correlate of protection is something that you can measure that tells you how well you are protected but at present we have none. The easiest thing to measure is IgG blood levels and there are plenty of tests for these but there is no clear way of interpreting them. Even if you measure neutralising antibodies it is of limited use as it is only one part of the immune response and not necessarily the most important. CD8 T cells, which can directly kill infected cells, are far more difficult to measure. There is also ADCC a process in which IgG, to any peptide from any viral protein, that is bound to the cells MHC receptors can cause NK cells to kill that cell. In a similar manner the complement cascade can puncture the cell membrane and induce apoptosis. All of these are part of the adaptive immune response and the efficiency on the innate response also needs to be allowed for.
The rapid antigen lateral flow test usually target the N protein which has 3 changes and 3 deletions and does not seem to have been compromised. The PCR tests normally use 3 primers only one of which targets a section of the spike sequence and this is causing a lower response (an S drop-out) but the other two work and it is reasonably simple to change the S primer so it works. The only practical effect is when the S drop-out is detected that sample is more likely to be selected for full sequencing which will give a false impression of how quickly Omicron is supplanting Delta. Covariants.org show the relative frequency of each variant by country in two week blocks based on GISAID submissions and is showing 100% Omicron in South Korea. A closer look shows it is based on one sequence, South Africa is 83% on 476 submissions but due to the S drop-out this is probably an over estimation. The best country to get data from will be the UK as it has the highest full sequencing rate typically submitting 100,000 in the same time period. The problem is they also have a very high vaccination rate and the highest rate of infected per head of population for countries over 20m. Under these conditions Omicron will have a big advantage over more ‘standard’ variants so, while accurate, may be a poor guide to relative fitness in other populations.
There has been speculation that virulence may be reduced but this is premature. For any reliable data we need to wait at least a month from the time there is a significant number of infections in any given population. We also need this from both a country like the UK and from an area with both low vaccination and previous infection rates.
There is a lot to consider and a lot I have left out.Last edited by JJackson; December 14, 2021, 06:55 PM.
Leave a comment:
-
concerning the waning effecitveness of the current COVID-vaccines,
I found this nice review published 2021-04-01: (see p.12)
(after checking keywords wane,waning in the first 20 out of 172 papers at google-scholar
found by keywords
waning covid vaccine novavax moderna mrna adenovirus
cited by 6 :
https://scholar.google.de/scholar?ci...iodt=0,5&hl=de
Nano-Enabled COVID-19 Vaccines: Meeting the Challenges of Durable Antibody Plus Cellular Immunity and Immune Escape
André E. Nel* and Jeff F. Miller
--------------------------------------
The neutralizing antibody response to seasonal
(“cold”) coronaviruses is of transient duration, allowing the
occurrence of reinfections.[33] In contrast, the protective
antibody responses to SARS-CoV-1 and MERS lasted a
minimum of 2−3 years after recovery.
...half-life of anti-RBD antibody decline to be ∼36 days;135
...people with milder infections generate lower antibody titers
that decline more rapidly.[138]
... 8 months {followup study}.139
{ neutralizing IgG antibody titers against the
spike protein and RBD remained relatively stable, with only a
modest decline over 6−8 months }
memory CD4+ and CD8+T-cells {have} half-lives of 3−5 months.
The decay kinetics of memory T-cell responses after
COVID-19 are similar to the vaccination response to the
yellow fever virus, which is known to confer long-lasting
immunity.[29]
there is a critical requirement for TFH
cooperation with germinal center B-cells in the development
of durable immunity to polio, smallpox, and other
a promising approach for augmenting memory B-cell
responses in COVID-19 could be to develop vaccinating
nanoparticles ...
Leave a comment:
-
Interestingly, a few new papers that expand on the potential risks here. I would suggest a MERS vaccination for camels asap would be prudent. Cats would seem to be less of a problem for us than we are to them.
1.https://www.atlantis-press.com/journals/jegh/125946349
2.https://academic.oup.com/jid/advance...iab104/6144906
3.https://www.jbc.org/article/S0021-92...208-8/fulltext
-
You have a wealth of information about the immune system organized here. Even though I don't grasp this as well as you, my sense is that you are right about the variant threat - there isn't a biological basis for fear at this point.
I don't fear a threat from other species as intermediates. I would think that if a human origin virus adapts to another species, it would lose its ability to spread easily among humans. That seems to be the usual pattern with farmers getting swine flu's. It is a risk we take to benefit financially or socially from relationships with other animal species.
Chinese scientists are doing some cruel experiments with dogs and flu, justifying this by claiming dogs might be mixing vessels.
-
gs I do not think there is a list of the type you want but the antigenic sites and the mAbs that react with them in the table in post #9 were selected because these are the serum antibodies that are most common. There are 12 in the table which would be a good start to your list. The IgG is about 150kDa where as the Spike is nearer 800kDa and, as it is a glycoprotein, you would need to add the weight of the glycans (just to get a sense of scale). I do not know if it will form clumps with SARS-CoV-2, as you would see in a agglutination assay, where the two branches of the IgG bind to spikes on two virions which then clump with others using more IgG bridges until you get a raft in which none of the virions able to infect a cell. The exposed Fc portion of the antibody will also bind other immune cell receptors which can then induce clumping by a similar bridging mechanism. This is not an uncommon mechanism.
-
> the most important 15 antibodies
is there a list ? I'd like to have the sequences
------------------------------
-----------------------------------------------------
Evolution of antibody immunity to SARS-CoV-2
received Nov3 , published Jan18 , Nature , 30 pages .pdf
they test 122 ("monoclonal"?) antibodies
they assign Cxxx numbers to them, e.g. C144
V367F,S477N,N439K,V483A,N440K,RBD ,R346S,A475V,E484K,Q493R
figure 3a) 122 selected monoclonal antibodies
figure 3b) 52 antibodies
figure 3e) 26 antibody clonal pairs
C098,C099 ; C202,C542 ; C032,C080 ; C132,C512 ; C108,C573
C564,C546 ; C151,C062 ; C148,C060 ; C091,C092 ; C044,C045
C548,C549 ; C114,C571 ; C005,C043 ; C143,C055 ; C164,C055
C089,C090 ; C058,C059 ; C058,C057 ; C085,C086 ; C144,C051
C021,C097 ; C554,C555 ; C002,C095 ; C144,C052 ; C144,C050
C144,C053
=============================================
comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding
domain that affect recognition py polyclonal human serum antibodies
Jesse D.Bloom et.al. , Jan04, BioRxiv , 35 pages .pdf
most important is E484
figure 6B)
S477N,N439K,N501Y,Y453F
E484K,K417N,S494P,L452R,G446V,F490S,L452M,L455F,E4 84Q,F486L,G485R
zero hits for 144 or monoclonal (mAb)
=============================================
definitions :
a monoclonal antibody (mAb or moAb) is an antibody made by cloning a
unique white parent blood cell
----------
polyclonal antibodies (pAbs) are antibodies that are secreted by
different B cell lineages within the body. They are a collection of
immunoglobulin molecules that rreact against a specific antigen,
each identifying a different epitope.
-----------
an antibody (Ab) also known as an immunoglobulin (Ig) is a
large Y-shaped protein used by the immune system to identify and
neutralize foreign objects such as pathogenic bacteria and viruses.
The antibody recognizes a unique molecule of the pathogen called an antigen.
-----------------
an antigen (Ag) is a molecule or molecular structure such as may be
present on the outside of a pathogen (spike?), that can be bound by an
antigen-specific antibody or B-cell antigen receptor.
(# I assume the antibody binds to one of the spikes and the whole virus
together with its other 20-100 spikes hangs at it)Last edited by gsgs; February 20, 2021, 12:38 AM.
-
Thank you for your interesting post. In terms of the final paragraph above, the study linked below provides some interesting (and concerning) insights. If this study is correct, it is not just mink and cats we need to be concerned about, but pigs. See https://www.nature.com/articles/s414...04MUUzWa2XuZRM. The linkage to C. Dromedar is also concerning, in view of MERS
Leave a comment:
-
Having looked a little at the Spike?s antigenic sites and the antibody interactions with it this post is going to look at the B cells that produce these antibodies.
Again I will start with some graphics which will help with the discussion.
In the diagram above we can see the 8 step process that takes a stem cell in the bone marrow through to the mature B cell in the Lymph nodes. This is not the main focus of this post but I will run through it briefly. The first 6 steps occur in the bone marrow and the last two in the Lymph nodes. From left to right the stromal cell binds to a receptor on the B cell causing it to produce a second receptor type which also binds causing IL7 release from the stromal cell initiating the production of the membrane bound antibody in the pre cell. This is further refined in the Immature cell which then travels to the secondary lymph nodes to complete the process.
Before we get to the antigen dependent part of the process it is worth looking a little at these last two steps in which the Ig light and heavy chains are added. Complex multicellular organisms, like us, can?t evolve on the same time scale as viruses so need a mechanism to quickly react to novel pathogens with which they have no prior experience ? like SARS-2. Nature?s solution is to make antibodies which have a fairly common fc portion, which is the trunk of the ?Y? shaped receptor in the diagram, and a variable short light and heavy chains. It achieves this by having thousands of these short gene sequences in our DNA for both the light and heavy chains the combinations of which can produce millions of different antibodies. Each B cell produces one of these millions most of which will die without ever having encountered a matched antigenic site. In an individual who has not suffered any recent antigenic challenge they still have about 80% of the number of these random B cells as someone fighting an infection. All these are just circulating waiting for their chance to interact with their matched antigen. In the unlikely event that this happens they then mature and start to rapidly divide and produce and release the antibodies which have the same heavy & light chain combination they presented on their surface. The first dose in a prime boost vaccine initiates this process and the second dose is timed to repeat it. The second dose instead of only having one in a million chance of interacting with a matched antibody the rapid multiplication of the original matched B cell has produced thousands more matched cells. All of these are ready to be activated and start the production of vast quantities of matched antibodies and yet more B cells to produce them.
The rest of this post is going to look at how that original light chain / heavy chain combination, which is close enough to the antigenic site to bind but probably not very strongly, is refined to give a better and better match. Again some graphics depicting some important players and sites.
The dendritic cells are a key player but only the green half of the image is relevant to our discussion. They are the body?s garbage collectors and are attracted to any sites of infection. Their octopus like shape allows them to squirm between tissues and on their surface they have MHC 1 & 2 receptors. They collect any bits of protein or nucleic acid they encounter and display them on their MHC cells. The Dendritic cells will then return to the lymph nodes and present the collected fragments to B and T cells. If their surface antibodies are a match for the presented antigens they will start to divide. T cells have a very similar heavy and light chain to the B cells and fall into two main groups, depending on their surface receptors, those with CD8 receptors target and kill infected cells presenting peptides that match their antibodies. The CD4 presenting cells are more relevant to the processes being discussed as they can become follicular helpers cells (Tfh). Most cells do have MHC1 receptors on their surface, which present very short pathogen peptides, due to their length they are not particularly specific and the matched CD8+ cytotoxic T cell?s will bind to them and kill the cells but upon infection they will also start making MHC2 receptors which present a slightly longer and more specific peptide presenting a higher bar to recognition. It is these that the CD4+ T cells recognise and dendritic cells always have MHC2, as well as MHC class 1, this allows them to become follicular helper cells which can then regulate the process of somatic hypermutation in the lymph node germinal centres. While the body may not care too much about CD8+ T cells killing a few cells that did not have exactly the right peptide on their MHC1?s it is fussier about making miss-targeted Tfh?s as it does not want to ramp up production of miss-matched antibodies ? hence the slightly higher bar set by the longer peptides presented by MHC2 cells.
The next two graphics show the lymphatic system and the germinal centres which are found within them.
The germinal centres have two zones, the light and the dark, in the light zone the follicular dendritic cell (FDC) can be seen with the red dots representing the MHC sites and the collected peptides with a B cell?s light and heavy chains bound to them. To the right of the B cell is a Tfh CD4+ cell which must also have the correct activation site. If both conditions are met the B cell can enter the dark zone where an enzyme is added that causes rapid mutation (hyper-mutation) of the light and heavy chains sequences which are then re-tested against the Tfh. Any with a low binding affinity die but those which have improved their bind go on to be reproduced. This process is repeated again and again against whatever peptides the dendritic cells have found and presented.
If we consider how this relates to a natural SARS-CoV-2 infection a cell is infected and presents viral peptides on the cell?s MHC1 surface protein. Millions of B and T cells interact with it until that one in a million match finds it and binds and becomes activated. The cell also begins MHC2 production which can bind and start the CD4 T cell activation turning it into a Tfh. The B cells will start antibody production but of relatively poorly matched IgM antibodies. The B cells then refine the antibody in the germinal centres and switch to a better targeted IgG antibody which is then fine-tuned in the dark zone until an even better binding strength is achieved. At this point the B cell can take two paths either it turns in to a very long lived Memory B cell or it turns into an effector cell, or plasma cell. The effector cells produce antibodies during the acute phase of the disease and then die off. The plasma cell becomes much larger and is packed with rough endoplasmic reticulum which produces vast quantities of protein for export from the cell. These proteins are the matched IgG antibodies and they have so much rough ER they can churn out 2000 IgG per second. As long as there is some viral fragments left somewhere in the body the dendritic cells will find them and present it in the lymph nodes to help the B and Tfh cell refine the IgG affinity.
If we now consider what would happen if you had been vaccinated, or had a natural infection, to one strain of the virus but then get challenged at a later date by a variant of that virus. This is only likely to have a few changes some of which reduce the binding affinity as we saw in the earlier post looking at weakened Mab to RBD binding. This time most of the antibodies work fine but some may not be as good as they were. The unaffected ones will give you a good level of protection while the process of somatic hyper mutation and Tfh matching for high affinity fits will start to produce new better fits to the changed antigenic sites. You are likely not to be symptomatic while this is occurring as the unchanged antigenic site / antibody interaction across all the unaffected sites give adequate protection while the germinal centres go about refining the one or two sites that had become less well paired.
Finally I will get much more speculative and give some thoughts on what I think is going to happen next in the battle between SARS-2 and us. With the Northern hemisphere winter we have seen clear evidence that this respiratory virus has a strong seasonality. As we head out of this season cases should drop which will be further strengthened by the increasing level of herd immunity induced by the combination of already infected plus those vaccinated. Sadly this will highlight the discrepancy between rich and poor nations as only the former will initially have much of a vaccine component. Over the past year there has been a continuous process of single nucleotide mutation (SNPs) most of which have had little impact on spread and either died out or continued to circulate at low levels. As more people have some level of immunity changes that were neutral, and at low levels, now convey a selective advantage if they weaken the neutralising effect of important antibodies. This is going to cause these changes to suddenly begin to take off and become dominant. This has nothing to do with increased virulence or transmissibility ? although if any of those changes do change either then it may impact them either up or down ? but that is a secondary effect.
The hypermutation can cope with gradual change but not massive jumps that cause many of the key antibodies to stop working effectively. In the 2009 flu pandemic, although many of us had antibodies to seasonal H1N1 (H & N being the two spikes which carry the antigenic sites to which the neutralising antibodies bind), the new strain had entirely new H1 & N1?s from another species which were sufficiently different for our immune system to view it as a new virus. This is likely to also happen for SARS. Although corona viruses only have one RNA strand and cannot therefore achieve this by recombination there is a reservoir in bats which is likely to cause a SARS 3 & 4 at some stage. The fact that SARS-2 has been transported all over the world by humans allowing it to interact with many new species like mink, dogs, cats etc. it is quite likely it will also set up shop in a novel species where it can adapt to that host for a while attaining enough changes to be viewed as new if it jumps back into us. This is the bigger risk in my opinion as these prospective host species have more interactions with humans and are physiologically closer to us than bats so a host optimised mink strain is likely to be better intermediate host as it started from a human optimised form and would probably retain a respiratory infection path rather than oral/fecal.
This was long but is a simplified explanation and only covers a small part of the whole immune response but it is important in understanding why the variants are not the doomsday scenario the MSM has been depicting.
Again if you see errors please point them out so I can make corrections.Last edited by JJackson; May 21, 2021, 07:22 AM.
Leave a comment:
-
MIS kids are very low in antibodies for common coronaviruses.
Here's the study:
-
In the previous two post I looked at the S protein and some of the RBD antigenic sites and variant changes and how they affect binding.
This time I aim to think a little bit about these sites and the immune system.
If you look at the antibodies in sera from different individuals, post natural infection, you will find a lot of different antibodies and significant differences in their relative abundance between individuals. There are however a number of points along the RBD, and spike more generally, that are over represented generally both in quantity in one serum and frequency across all the sera. They are concentrated in more neutralising antibodies and surprise, surprise they closely correlate with those areas targeted by the MAbs which showed the most reductions in neutralisation in the table in post #9 e.g. S 417, 484 & 501.
What I am building up to is the hypothesis that these are occurring now because they are immune escape changes that effect a change at key antibody binding sites. If this is the case then the change would need to have a functional effect and it should become more prevalent as the number of potential hosts with pre-existing antibodies increases. What data is there and does it support this hypothesis?
If we take the E484K change, found both in South Africa and South America, the side chain changes its charge from – to +. If we look at how it has changed over time The graphics below show firstly how K has grown over time and then the full tree – green being E484 and yellow K484 - and finally recorded case of SARS-CoV-2 globally.
I have attempted to line up the dates on the X axis so they correspond.
The first will be hard to read but it should be possible to see some yellow by October but a closer examination shows it reached 1% by the first week in July and is now 14% (There were about 4.5m active cases in July so 1% would give 45,000 active infections carrying K484).
The tree in image 2 will not be readable but the things to note are the yellow clump near the top (S America) and the one nearer the bottom (S Africa) the smaller intermediate clump is mainly Nigeria. Also note they are all independent emergences in different clades and have been happening for a while but have only taken off since October. A closer examination of the top branch shows the first case in Brazil on the 9th of October but that the calculated date for the route of that branch is late July. On the S African branch the route is dated as early July with the first sequenced case on the 28th of October.
Moving to the bottom graph Mid July shows about 15 to 20 million cases which climbs to nearer 40 million by the first sequenced cases and then on to over a hundred million.
Brazil and South Africa have the most cases on their respective continents so will have a relatively high sero-prevalence rate. In Brazil they have about 5% of the population as PCR confirmed cases with that actual number probably 3 times that. In SA the percentage is 2.5% but its undercount is probably even greater. These numbers will be beginning to challenge the virus at any critical points that are no longer doing what the virus needs them to due to antibody interference. Any change at these points that does not carry too big a fitness penalty will now begin to establish itself as it now has a selective advantage at least in the previously infected. As the percentage of the population that have antibodies to E484 increases the K484 variant’s advantage will grow.
I will not go through all the others in detail but N501Y makes it more hydrophobic, there are 3 main unrelated branches with no sequences until late Oct. but routed back in the spring.
The sera from vaccinated individuals is much the same across the Spike protein but lacks all the other antibodies generated against the rest of genome. While these are not likely to induce many neutralising antibodies they will react to a subsequent challenge activating the their B and T cells which can release cytokines and, in the case of CD8+ cells, kill infected cells displaying the peptides they can recognise. This may not be as effective as stopping cell fusion but will stimulate the immune response generally. Only recognising antigenic sites on S means they can target the antibody response to the most effective sites but on the down side that is the least conserved part of the least conserved protein. Any change in these site will impact it disproportionally, compared to a natural infection, as it does not have any other antibodies to fall back on.
Single AA changes at any one point will only effect one or two of the most important 15 antibodies so without a radical change across many of these (which would probably render the RBD non-fuctional) it should give most of the protection against a variant you would have got to the strain the vaccine was based on. This protection should contain the virus to non-severe infection giving your immune system time to generates new antibodies to the changed antigens and boost all of the ones that had not changed. Under the scenario I have outlined the variants we have encountered to date are not a major concern even if they show reduced protection as long as they buy the patient time to redress the balance. I view the decline as much the same as the decline you are going to get anyway as time elapses from your last infection or vaccination.Last edited by JJackson; February 10, 2021, 06:48 PM.
Leave a comment:
-
Thanks to you both but just to be clear I am not a scientist just an interested layman.
I wish I had listened to TWiV 714 & 715 before writing the above post as they cover exactly this topic and I have learnt a lot of new information which has generated new questions.
Firstly it appears in addition to the pathway I described above there is another which is similar to that used by HIV. From the first post you may recall that HIV also employs Class 1 fusion but instead of the whole virion entering in an endosome, which it has to get out of as an additional step, the capsid releases its payload directly through the pore into the cytosol - SARS-2 can perform the same trick but SARS-1 couldn't or at least to the same degree. This finding comes out of research into why Hydroxychloroquine worked well in cell culture but not in a clinical settings. The answer lies in the transmembrane host protease TMPRSS2 which is a major cleaver of the furin S1/S2 site in SARS-2 but SARS-1 did not have a furin accessible site at this point. SARS-1 was susceptible to HCQ as were the vero cells used in cell culture which had the human ACE2 receptor but very few TMPRESS2. If you re-engineer the vero cells to display plenty of TMPRSS2 then the HCQ stops working. This and a raft of confirmatory experiments showed TMPRESS2 cleavage of the furin site at S1/S2 prior to S2' cleavage enables direct entry into the cytoplasm by bypassing the endosome. The graphic below shows both routes of entry are available.
In the first post I had said the S1/S2 cleavage was optional but helped, which is true as far as getting into the endosome, but it is obligatory at some point. For SARS-1 this happens in the endosome by another protease cathepsin-L. SARS-2 can use either route. Yet more experiments show HCQ is acting on the endosomal pathway and did not work with SARS-2 because it largely bypassed it.
The next image relates more to the first post but shows the positioning of the all the areas I discussed along the S chain although their proximity in the tertiary protein structure has little to do with how close they are here.
Edit:
I forgot the links.
714 is a conversation with Jason McLellan relating to the action of the Spike trimer and its cleavage sites.
 Jason McLellan joins TWiV to reveal all we know about the SARS-CoV-2 spike protein, followed by Novavax and J&S phase 3 results and a discussion of variants of concern: neutralization by vaccine-induced antibodies, transmission, and virulence.
Jason McLellan joins TWiV to reveal all we know about the SARS-CoV-2 spike protein, followed by Novavax and J&S phase 3 results and a discussion of variants of concern: neutralization by vaccine-induced antibodies, transmission, and virulence.
715 covers the HCQ inactivation and links it to an additional entry pathway avoiding HCQ's antiviral activity.
 TWiV explains why hydroxychloroquine failed in humans despite showing antiviral effects in cells, and reviews the published data on the Pfizer/BioNTech mRNA vaccine.
TWiV explains why hydroxychloroquine failed in humans despite showing antiviral effects in cells, and reviews the published data on the Pfizer/BioNTech mRNA vaccine.
The paper being discussed can be found here.
https://journals.plos.org/plospathog...l.ppat.1009212Last edited by JJackson; February 8, 2021, 04:01 PM.
Leave a comment:
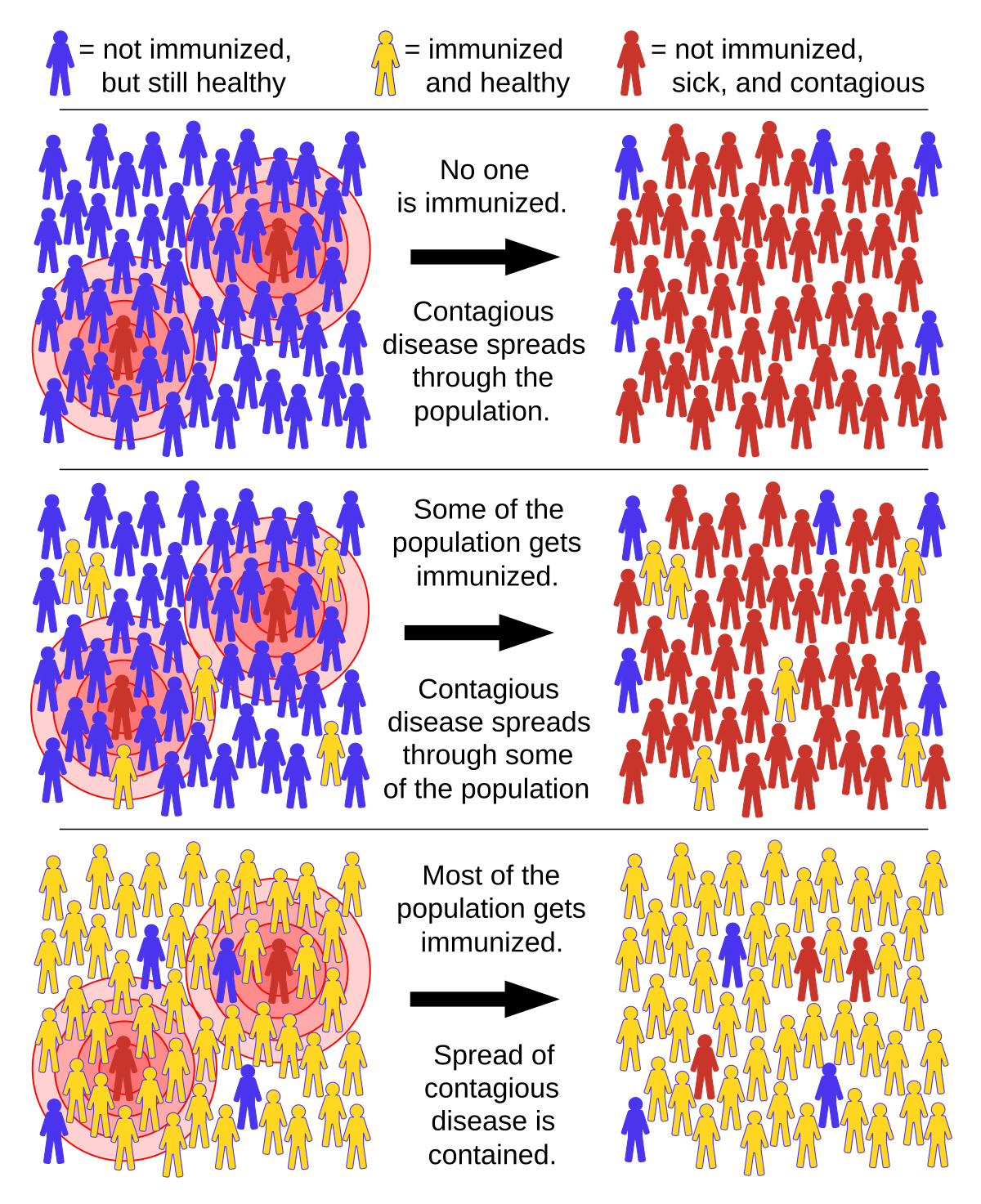
Leave a comment: