hat tip Hogvet51
snip
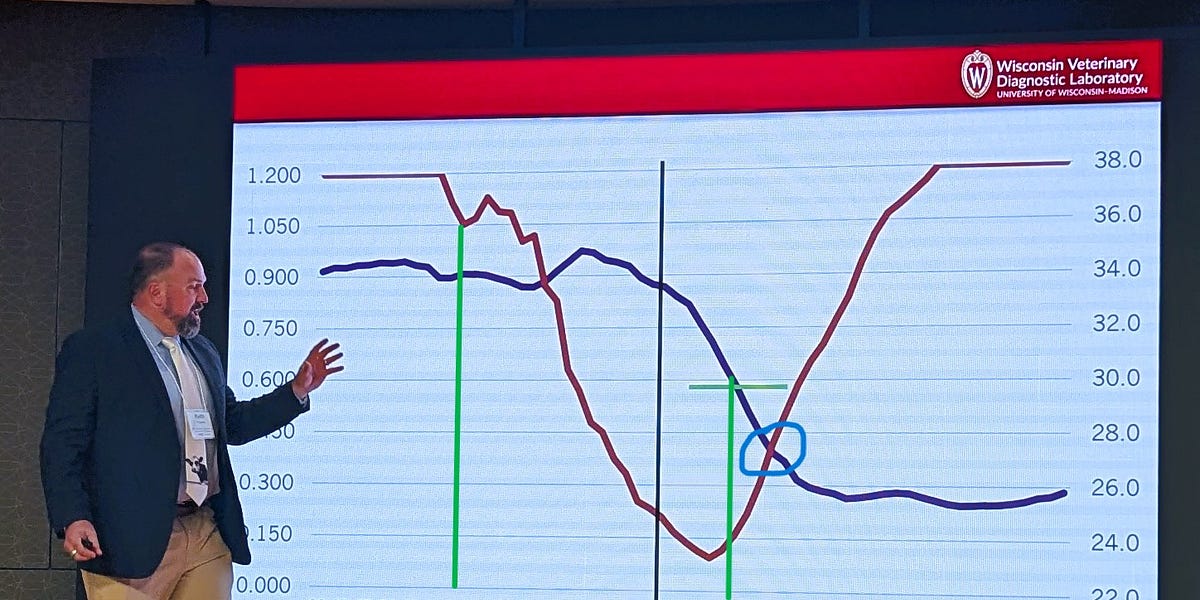
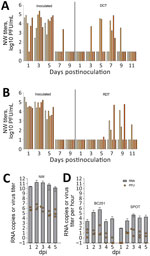
In summary we are at the point where the case load is slowing and released information is slowing even more as federal and state animal and public health staffs are reduced by cuts and less proactive U.S. public health policies come to the forefront. I don’t think our risk for cross species infections or for another human pandemic from H5N1 (or any other agent) has lessened whatsoever; however, our odds for early detection and a timely effective response continue to deteriorate daily.
I talked to an unnamed former VS colleague at the North Carolina meeting, still with a job, but quite pessimistic that VS or the animal health regulatory infrastructure in general has even minimal staff in place to handle a large resurgence in HPAI outbreaks next fall, let alone a major zoonotic pandemic or foreign animal disease response.
I hope industry is ready to step up to fend for itself in the event of a major animal health challenge, including one that involves a One Health threat. The capacity for a sustained federal or state effort will not be there initially. Plan accordingly to protect your interests.
For those of us who still believe, it may be a good time keep up our seasonal influenza vaccines for limited cross protection and to stockpile some N-95’s for those we love!








Comment