Re: Seasonal Flu 2008 - 2009
EISS - Inter-season Electronic Bulletin - Week 39 : 22/09/2008-28/09/2008 - 03 October 2008, Issue N. 273 - SPORADIC INFLUENZA ACTIVITY IN EUROPE
EISS - Inter-season Electronic Bulletin - Week 39 : 22/09/2008-28/09/2008 - 03 October 2008, Issue N. 273 - SPORADIC INFLUENZA ACTIVITY IN EUROPE
EISS - Inter-season Electronic Bulletin - Week 39 : 22/09/2008-28/09/2008 - 03 October 2008, Issue N. 273 - SPORADIC INFLUENZA ACTIVITY IN EUROPE
Summary:
Influenza virus detections occur very sporadically in Europe.
In week 38/2008, there were two detections and in week 39 there were nine detections reported.
Out of nine countries reporting the geographical spread indicator in week 38-39/2008, all reported no influenza activityIn weeks 38-39/2008, 11 influenza viruses were detected in Europe out of a total of 244 investigated specimens: one influenza A not subtyped virus each in Poland, Spain, Switzerland and Sweden, respectively, and four in England.
One type A(H3) in Germany, one type A(H3N2) and one type B in Sweden.
All detections were from non-sentinel specimens.
As usual for this time of the year, a number of the influenza cases are travellers who have been abroad.
There have been no reports of unusual influenza activity in Europe at community level (i.e. in a region or local area such as a city, county or district) since week 16/2008.
Background:
The Inter-season Electronic Bulletin presents and comments influenza activity based on virological data reported to EISS.
In weeks 38/2008 and 39/2008, a total of 16 countries reported virological data to EISS.
The Inter-season Electronic Bulletin will be published between week 21/2008 and week 39/2008.
The spread of influenza virus strains and their epidemiological impact in Europe are being monitored by EISS in collaboration with the WHO Collaborating Centre in London (United Kingdom) and the European Centre for Disease Prevention and Control in Stockholm (Sweden).
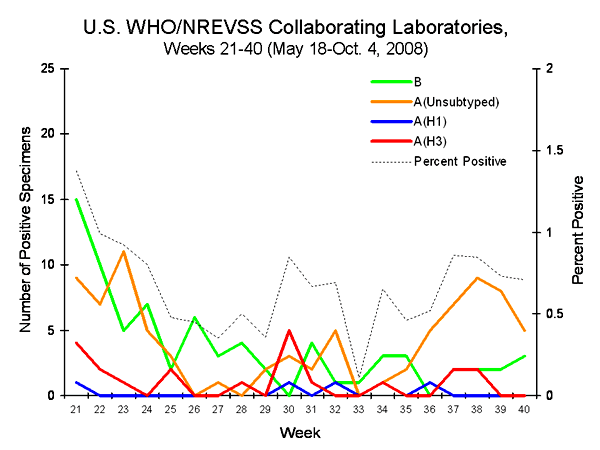
Graph
The graph presents the total number of specimens positive for influenza A and B viruses in Europe during the inter-season period.
<table style="width: auto;"><tbody><tr><td> </td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>
</td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>
Map
The map presents the geographical spread as assessed by each of the networks in EISS.
<table style="width: auto;"><tbody><tr><td> </td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>
</td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>
Europe, Year 2008 / Week 39
A = Dominant virus A
H1N1 = Dominant virus A(H1N1)
H3N2 = Dominant virus A(H3N2)
H1N2 = Dominant virus A(H1N2)
B = Dominant virus B
A & B = Dominant virus A & B
= : stable clinical activity
+ : increasing clinical activity
- : decreasing clinical activity
No activity = no evidence of influenza virus activity (clinical activity remains at baseline levels)
Sporadic = isolated cases of laboratory confirmed influenza infection
Local outbreak = increased influenza activity in local areas (e.g. a city) within a region,or outbreaks in two or more institutions (e.g. schools) within a region. Laboratory confirmed.
Regional activity = influenza activity above baseline levels in one or more regions witha population comprising less than 50% of the country's total population. Laboratory confirmed.
Widespread = influenza activity above baseline levels in one or more regions with a populationcomprising 50% or more of the country's population. Laboratory confirmed.
Finland : Where available, the epidemiological data are provided by a health-care district inSouth-Western Finland (the health-care district serves 54,000 inhabitants i.e. approximately onepercent of the Finnish population).
Network comments (where available)
* Sweden - The patient with Influenza A has been in London, the Influenza B patient in Senegal.
--
<cite cite="http://www.eiss.org/cgi-files/bulletin_v2.cgi">EISS - Bulletin Review</cite>
Summary:
Influenza virus detections occur very sporadically in Europe.
In week 38/2008, there were two detections and in week 39 there were nine detections reported.
Out of nine countries reporting the geographical spread indicator in week 38-39/2008, all reported no influenza activityIn weeks 38-39/2008, 11 influenza viruses were detected in Europe out of a total of 244 investigated specimens: one influenza A not subtyped virus each in Poland, Spain, Switzerland and Sweden, respectively, and four in England.
One type A(H3) in Germany, one type A(H3N2) and one type B in Sweden.
All detections were from non-sentinel specimens.
As usual for this time of the year, a number of the influenza cases are travellers who have been abroad.
There have been no reports of unusual influenza activity in Europe at community level (i.e. in a region or local area such as a city, county or district) since week 16/2008.
Background:
The Inter-season Electronic Bulletin presents and comments influenza activity based on virological data reported to EISS.
In weeks 38/2008 and 39/2008, a total of 16 countries reported virological data to EISS.
The Inter-season Electronic Bulletin will be published between week 21/2008 and week 39/2008.
The spread of influenza virus strains and their epidemiological impact in Europe are being monitored by EISS in collaboration with the WHO Collaborating Centre in London (United Kingdom) and the European Centre for Disease Prevention and Control in Stockholm (Sweden).
Graph
The graph presents the total number of specimens positive for influenza A and B viruses in Europe during the inter-season period.
<table style="width: auto;"><tbody><tr><td>
 </td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>
</td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>Map
The map presents the geographical spread as assessed by each of the networks in EISS.
<table style="width: auto;"><tbody><tr><td>
 </td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>
</td></tr><tr><td style="font-family: arial,sans-serif; font-size: 11px; text-align: right;">From TABLES</td></tr></tbody></table>Europe, Year 2008 / Week 39
A = Dominant virus A
H1N1 = Dominant virus A(H1N1)
H3N2 = Dominant virus A(H3N2)
H1N2 = Dominant virus A(H1N2)
B = Dominant virus B
A & B = Dominant virus A & B
= : stable clinical activity
+ : increasing clinical activity
- : decreasing clinical activity
No activity = no evidence of influenza virus activity (clinical activity remains at baseline levels)
Sporadic = isolated cases of laboratory confirmed influenza infection
Local outbreak = increased influenza activity in local areas (e.g. a city) within a region,or outbreaks in two or more institutions (e.g. schools) within a region. Laboratory confirmed.
Regional activity = influenza activity above baseline levels in one or more regions witha population comprising less than 50% of the country's total population. Laboratory confirmed.
Widespread = influenza activity above baseline levels in one or more regions with a populationcomprising 50% or more of the country's population. Laboratory confirmed.
Finland : Where available, the epidemiological data are provided by a health-care district inSouth-Western Finland (the health-care district serves 54,000 inhabitants i.e. approximately onepercent of the Finnish population).
Network comments (where available)
* Sweden - The patient with Influenza A has been in London, the Influenza B patient in Senegal.
--




Comment