High Blood Lead Levels in Children Consuming Recalled Cinnamon Applesauce Pouches
Print


Distributed via the CDC Health Alert Network
November 13, 2023, 2:00 PM ET
CDCHAN-00500
Summary
Multiple states have reported potential cases to the U.S. Food and Drug Administration (FDA) of high blood lead levels (BLLs) in children consuming recalled cinnamon-containing applesauce products that have high levels of lead. The Centers for Disease Control and Prevention (CDC) is issuing this Health Alert Network (HAN) Health Advisory to advise clinicians and health departments to consider the possibility of illness due to lead exposure and report cases to their local health authorities.
Background
FDA, CDC, and state and local partners are investigating a potential link between high BLLs and consuming certain cinnamon-containing apple purée and applesauce products.
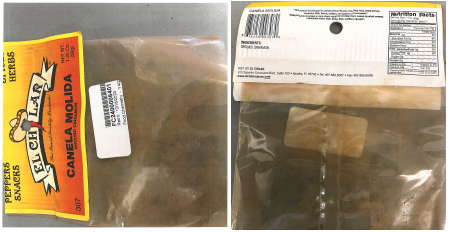
State partners tested multiple lots of the reported products, and test results indicated the products contained extremely high levels of lead. WanaBana, Schnucks, and Weis have initiated voluntary recalls of certain lots of the following products:
More information about the specific recalled products may be found on the FDA’s website: Investigation of Elevated Lead Levels: Applesauce Pouches (November 2023) | FDA
As of November 7, 2023, there are 22 cases, in states including Alabama, Arkansas, Louisiana, Maryland, Missouri, New Mexico, New York, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, Texas, and Washington, ages 1 to 3 years, with BLLs ranging from 4 to 29 micrograms per deciliter (µg/dL). Cases experienced signs and symptoms including headache, nausea, vomiting, diarrhea, change in activity level, and anemia.
No safe level of lead in children’s blood has been identified. CDC does not use the term “elevated blood lead levels” when recommending what actions to take based on a child’s blood lead level (BLL). CDC uses a blood lead reference value (BLRV) of 3.5 µg/dL to identify children with BLLs that are higher than most children’s levels. The BLRV is based on the 97.5th percentile of the BLLs among U.S. children ages 1–5 years. The BLL can be obtained using a capillary or venous blood draw. Capillary lead levels ≥3.5 µg/dL require confirmatory testing with a venous blood level to rule out contamination. Children who have eaten the recalled products or have other suspected sources of lead exposure should be tested.
Lead toxicity primarily targets the central nervous system. Children are more vulnerable to lead poisoning than adults because their nervous systems are still developing. Children also tend to absorb a higher fraction of ingested lead than adults. Although children with lead exposure may have no apparent acute symptoms, even low levels of lead have been associated with learning, behavioral, and cognitive deficits. A child who is exposed to large amounts of lead may develop acute lead poisoning, presenting with gastrointestinal, hematological, and neurological effects, including one or more of the following signs and symptoms: anemia, abdominal pain, weakness, and severe neurological sequelae (e.g., seizures, encephalopathy, and coma), which may result in brain damage. Some effects of lead poisoning in a child may continue into adulthood. Adults who have high BLLs may be at increased risk for high blood pressure, other cardiovascular effects, kidney problems, adverse reproductive outcomes, and gout. More information about adverse effects of lead exposure can be found in the ATSDR Lead Toxicological Profile.
Managing acute lead poisoning includes eliminating the exposure, providing supportive and symptomatic care, and quantifying lead exposure by checking BLLs. Children who are symptomatic with elevated BLLs above 45 µg/dL may require hospital admission for monitoring and chelation therapy using medications such as succimer, dimercaprol, or edetate calcium disodium (EDTA). Healthcare providers can find recommendations on management of childhood lead exposure and other resources on the Pediatric Environmental Health Specialty Units website.
Recommendations for Clinicians
Recommendations for Public Health Professionals
Recommendations for the Public (Parents, Caregivers, Guardians)
For More Information
References


Distributed via the CDC Health Alert Network
November 13, 2023, 2:00 PM ET
CDCHAN-00500
Summary
Multiple states have reported potential cases to the U.S. Food and Drug Administration (FDA) of high blood lead levels (BLLs) in children consuming recalled cinnamon-containing applesauce products that have high levels of lead. The Centers for Disease Control and Prevention (CDC) is issuing this Health Alert Network (HAN) Health Advisory to advise clinicians and health departments to consider the possibility of illness due to lead exposure and report cases to their local health authorities.
Background
FDA, CDC, and state and local partners are investigating a potential link between high BLLs and consuming certain cinnamon-containing apple purée and applesauce products.
State partners tested multiple lots of the reported products, and test results indicated the products contained extremely high levels of lead. WanaBana, Schnucks, and Weis have initiated voluntary recalls of certain lots of the following products:
- WanaBana brand apple cinnamon fruit purée pouches
- Schnucks brand cinnamon applesauce pouches
- Weis brand cinnamon applesauce pouches
More information about the specific recalled products may be found on the FDA’s website: Investigation of Elevated Lead Levels: Applesauce Pouches (November 2023) | FDA
As of November 7, 2023, there are 22 cases, in states including Alabama, Arkansas, Louisiana, Maryland, Missouri, New Mexico, New York, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, Texas, and Washington, ages 1 to 3 years, with BLLs ranging from 4 to 29 micrograms per deciliter (µg/dL). Cases experienced signs and symptoms including headache, nausea, vomiting, diarrhea, change in activity level, and anemia.
No safe level of lead in children’s blood has been identified. CDC does not use the term “elevated blood lead levels” when recommending what actions to take based on a child’s blood lead level (BLL). CDC uses a blood lead reference value (BLRV) of 3.5 µg/dL to identify children with BLLs that are higher than most children’s levels. The BLRV is based on the 97.5th percentile of the BLLs among U.S. children ages 1–5 years. The BLL can be obtained using a capillary or venous blood draw. Capillary lead levels ≥3.5 µg/dL require confirmatory testing with a venous blood level to rule out contamination. Children who have eaten the recalled products or have other suspected sources of lead exposure should be tested.
Lead toxicity primarily targets the central nervous system. Children are more vulnerable to lead poisoning than adults because their nervous systems are still developing. Children also tend to absorb a higher fraction of ingested lead than adults. Although children with lead exposure may have no apparent acute symptoms, even low levels of lead have been associated with learning, behavioral, and cognitive deficits. A child who is exposed to large amounts of lead may develop acute lead poisoning, presenting with gastrointestinal, hematological, and neurological effects, including one or more of the following signs and symptoms: anemia, abdominal pain, weakness, and severe neurological sequelae (e.g., seizures, encephalopathy, and coma), which may result in brain damage. Some effects of lead poisoning in a child may continue into adulthood. Adults who have high BLLs may be at increased risk for high blood pressure, other cardiovascular effects, kidney problems, adverse reproductive outcomes, and gout. More information about adverse effects of lead exposure can be found in the ATSDR Lead Toxicological Profile.
Managing acute lead poisoning includes eliminating the exposure, providing supportive and symptomatic care, and quantifying lead exposure by checking BLLs. Children who are symptomatic with elevated BLLs above 45 µg/dL may require hospital admission for monitoring and chelation therapy using medications such as succimer, dimercaprol, or edetate calcium disodium (EDTA). Healthcare providers can find recommendations on management of childhood lead exposure and other resources on the Pediatric Environmental Health Specialty Units website.
Recommendations for Clinicians
- Counsel patients or their caregivers and guardians not to eat specific cinnamon-containing apple purée or applesauce products named in the FDA recall announcements.
- Educate patients or their caregivers and guardians about the health effects of lead exposure in children and the importance of seeking medical care. Most children have no obvious symptoms, but appropriate screening can detect lead in blood. Children who have consumed a recalled applesauce pouch product should be tested for lead exposure. Clinicians may refer to CDC’s guidance on testing children for lead exposure. The American Academy of Pediatrics has also published clinical guidance for managing lead exposure in children. More specific recommendations for obtaining BLLs in your jurisdiction may be available from your local health department or regional Pediatric Environmental Health Specialty Unit (PEHSU).
- Consider lead exposure in the differential diagnosis of patients presenting with compatible clinical findings associated with lead poisoning, which may include the following:
- Constitutional symptoms such as generalized weakness, fatigue, malaise, arthralgias, myalgias, irritability, anorexia, insomnia, and weight loss.
- Abdominal pain (“lead colic”), constipation, nausea, and other gastrointestinal symptoms.
- Anemia (normochromic or microcytic, possibly with basophilic stippling).
- Central nervous system effects, such as headache, impaired visual-motor coordination, tremor, and, in severe cases, seizure, encephalopathy, and coma.
- Stunted growth, hearing problems, impaired neurobehavioral development, decreased intelligence, and failure to meet expected developmental milestones.
- Impaired kidney function, such as acute tubular dysfunction.
- Obtain a detailed exposure history in patients with suspected lead exposure, including those who consumed a recalled product. Also, ask about household members with known lead exposures and possible lead sources in and around the home. Parents and caregivers who work in jobs, hobbies, or other activities that expose them to lead can bring lead-containing dust home with them. Lead-containing dust can be tracked onto carpets, floors, furniture, and other surfaces that a child may touch, and expose other family members without knowing. Known risk-factors for lead exposure include the following:
- Lead paint and dust in homes built before 1978.
- Lead in soil, for example due to prior contamination from leaded gasoline, exterior lead paint, or old home renovations.
- Nearby active lead and other types of smelters, battery recycling plants, or other industries that release lead into the air,
- Certain folk remedies (e.g. Ayurvedic or traditional Chinese herbal medicines, Azarcon, Greta), cosmetics (e.g. kohl, kajal, surma), religious powders (e.g. sindoor), and other cultural products.
- Imported powdered spices, such as turmeric, chili, and curry powders.
- Certain types of jewelry made with lead-containing metal alloys or paints.
- Ceramics made with lead-containing glazes.
- Older toys made with lead-based paint, lead-containing metal alloys, or certain types of plastic.
- Know that individuals with high BLLs may not be symptomatic and are identified through screening. Be familiar with CDC’s testing recommendations for lead, indications for confirmatory testing, and recommended actions based on BLL. CDC recommends a blood lead reference value (BLRV) of 3.5 µg/dL to identify children with BLLs that are higher than most.
- Obtain early consultation with or provide a referral to a medical toxicologist or pediatric specialist with expertise in managing lead exposure for medical workup and managing patients with high BLLs.
- Contact your local health authority to report cases of individuals with BLLs above the reference value, including those who have consumed these recalled products.
- Contact your local poison center (1-800-222-1222) for advice on diagnosing and managing lead toxicity.
Recommendations for Public Health Professionals
- Know that individuals with high BLLs may not be symptomatic. Case finding may be mainly from reporting by clinicians who recognize risks of exposure and perform screening.
- Consider conducting case-finding activities that leverage existing data sources such as medical encounter and hospital discharge data, electronic syndromic surveillance systems, your local poison center, and other applicable surveillance systems.
Recommendations for the Public (Parents, Caregivers, Guardians)
- Do not buy, eat, sell, or serve recalled cinnamon-containing applesauce pouch products because they may contain lead.
- Parents and caregivers of children who may have consumed recalled products should contact the child’s healthcare provider about getting a blood test for lead.
For More Information
- FDA
- CDC/ATSDR
- America’s Poison Centers
- Pediatric Environmental Health Specialty Units
- American College of Medical Toxicology
References
- Agency for Toxic Substances and Disease Registry. Toxicological profile for lead. Atlanta, GA: U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; 2020. https://wwwn.cdc.gov/TSP/ToxProfiles...x?id=96&tid=22
- CDC. Lead Exposure and Prevention Advisory Committee (LEPAC) meeting [transcript]. Atlanta GA: U.S. Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/nceh/lead/advisory/docs/LEPAC-transcript-10-30-20-508.pdf
- CDC. Recommended actions based on blood lead level. Atlanta GA: US Department of Health and Human Services, CDC; 2021. https://www.cdc.gov/nceh/lead/adviso...tions-blls.htm
- Egan KB, Cornwell CR, Courtney JG, Ettinger AS. Blood lead levels in U.S. children ages 1–11 years, 1976–2016. Environ Health Perspect 2021;129:37003. https://doi.org/10.1289/EHP7932 PMID:33730866
- Ruckart PZ, Jones RL, Courtney JG, LeBlanc TL, Jackson W, Karwowski MP, Cheng P, Allwood P, Svendsen ER, Breysse PN. Update of the Blood Lead Reference Value — United States, 2021. MMWR. 2021; 70(43):1509–1512.











Comment