Eurosurveillance: Human Infections with Eurasian Avian-like Swine Influenza Virus Detected by Coincidence Via Routine Respiratory Surveillance Systems, the Netherlands, 2020 to 2023
#18,720
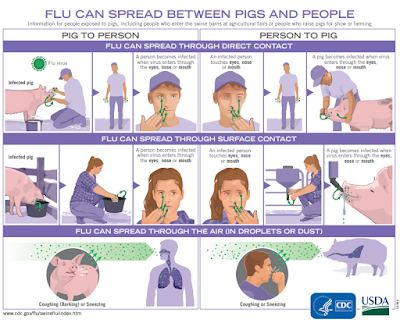
Conventional wisdom holds that swine variant flu infections don't transmit well in humans, and that most of the (rare) cases reported are due to exposure to pigs; usually on farms or at agricultural exhibits.
While largely true, there are a few caveats. Primarily that swine variant infections are generally mild, and are undisguisable from seasonal colds or flus without specialized laboratory testing. Something that is rarely done for flu patients who are mildly ill.
Over the years we've seen estimates that the cases we see are likely only the tip of the iceberg. A dozen years ago, during a small outbreak (n=13) of H3N2v in the United States - researchers estimated that fewer than 1 in every 200 cases was identified (see CID Journal: Estimates Of Human Infection From H3N2v (Jul 2011-Apr 2012).
Results. We estimate that the median multiplier for children was 200 (90% range, 115–369) and for adults was 255 (90% range, 152–479) and that 2055 (90% range, 1187–3800) illnesses from H3N2v virus infections may have occurred from August 2011 to April 2012, suggesting that the new virus was more widespread than previously thought.
We've seen similar estimates with other novel viruses, including H7N9 in China and MERS-CoV in Saudi Arabia, while seroprevalence studies have indicated that spillovers of H9N2 - and even H5N1 - often go unreported.
While there is no evidence of sustained transmission of swine variant influenza, we have seen a significant number of sporadic cases which report no (direct or indirect) contact with pigs, which raises questions about how that person was exposed.
A little over a month ago, in Emerg. Microbes & Inf.: Eurasian 1C Swine Influenza A Virus Exhibits High Pandemic Risk Traits, we looked at growing concerns over the Eurasian avian-like swine influenza A(H1N1)v virus clade 1C.2.1, which has become endemic in pig populations in Europe and Asia.
They reported:
Since 2010, at least 21 spillover events of 1C virus into humans have been detected and three of these occurred from July to December of 2023.
and
Yesterday's Eurosurveillance reports on 3 swine 1C cases serendipitously detected through routine surveillance since 2020 - 2 of which had no direct contact with pigs - from the Netherlands. They key messages being:

The Eurosurveillance report is lengthy and informative, and worth reading in its entirety. I've posted the link and some excerpts below. I'll have a bit more when you return.
Human infections with Eurasian avian-like swine influenza virus detected by coincidence via routine respiratory surveillance systems, the Netherlands, 2020 to 2023
Dirk Eggink1, Annelies Kroneman1 , Jozef Dingemans2 , Gabriel Goderski1 , Sharon van den Brink1 , Mariam Bagheri1 , Pascal Lexmond3 , Mark Pronk3 , Erhard van der Vries5 , Evelien Germeraad4 , Diederik Brandwagt1 , Manon Houben5 , Mariëtte van Hooiveld6 , Joke van der Giessen1 , Ron Fouchier3 , Adam Meijer1
(SNIP)
(Continue . . . )
While avian flu currently gets the bulk of our attention due to the rapid evolution and spread of HPAI H5N1, we also keep close tabs on swine flu, because pigs are considered to be excellent reservoirs and `mixing vessels' for influenza.

While there are numerous swine-variants around the world, here in the United States the CDC has identified 3 as having some pandemic potential.
And of course, we now have increased concerns over the potential for avian H5N1 to spillover into pigs, as it already has with dairy cows. Thus far, only two pigs have tested positive in the United States, but elsewhere in the world we've seen sporadic reports of spillovers.
WHO H5N1 detected in pigs in China (2004)
EID Journal: Asymptomatic H5N1 In Pigs (2010)
An Unusual Report Of H5N1 in Pigs (Indonesia 2016)
Sci. Rpts.: Evidence Of H5N1 Exposure In Domestic Pigs - Nigeria (2018)
Seroconversion of a Swine Herd in a Free-Range Rural Multi-Species Farm against HPAI H5N1 2.3.4.4b Clade Virus (2023)
But the reality is, most of the world isn't even looking. Even here in the United States, testing of pigs for novel flu viruses is largely voluntary and anonymous. Since pigs are generally able to carry novel flu viruses (including H5N1) asymptomatically, passive surveillance is unlikely to detect infections.
While most of the emerging novel viruses we look at in this blog will probably never pose a genuine global health threat - there are a lot of contenders out there - and it only takes one overachiever to make an indelible mark on the world.
https://afludiary.blogspot.com/2025/...ions-with.html

#18,720
Conventional wisdom holds that swine variant flu infections don't transmit well in humans, and that most of the (rare) cases reported are due to exposure to pigs; usually on farms or at agricultural exhibits.
While largely true, there are a few caveats. Primarily that swine variant infections are generally mild, and are undisguisable from seasonal colds or flus without specialized laboratory testing. Something that is rarely done for flu patients who are mildly ill.
Over the years we've seen estimates that the cases we see are likely only the tip of the iceberg. A dozen years ago, during a small outbreak (n=13) of H3N2v in the United States - researchers estimated that fewer than 1 in every 200 cases was identified (see CID Journal: Estimates Of Human Infection From H3N2v (Jul 2011-Apr 2012).
Results. We estimate that the median multiplier for children was 200 (90% range, 115–369) and for adults was 255 (90% range, 152–479) and that 2055 (90% range, 1187–3800) illnesses from H3N2v virus infections may have occurred from August 2011 to April 2012, suggesting that the new virus was more widespread than previously thought.
We've seen similar estimates with other novel viruses, including H7N9 in China and MERS-CoV in Saudi Arabia, while seroprevalence studies have indicated that spillovers of H9N2 - and even H5N1 - often go unreported.
While there is no evidence of sustained transmission of swine variant influenza, we have seen a significant number of sporadic cases which report no (direct or indirect) contact with pigs, which raises questions about how that person was exposed.
A little over a month ago, in Emerg. Microbes & Inf.: Eurasian 1C Swine Influenza A Virus Exhibits High Pandemic Risk Traits, we looked at growing concerns over the Eurasian avian-like swine influenza A(H1N1)v virus clade 1C.2.1, which has become endemic in pig populations in Europe and Asia.
They reported:
Since 2010, at least 21 spillover events of 1C virus into humans have been detected and three of these occurred from July to December of 2023.
and
The 1C virus exhibited phenotypic signatures similar to the 2009 pandemic H1N1 virus, including human receptor preference, productive replication in human airway cells, and robust environmental stability. Efficient inter- and intraspecies airborne transmission using the swine and ferret models was observed, including efficient airborne transmission to ferrets with pre-existing human seasonal H1N1 immunity. Together our data suggest H1 1C influenza virus poses a relatively high pandemic risk.
Yesterday's Eurosurveillance reports on 3 swine 1C cases serendipitously detected through routine surveillance since 2020 - 2 of which had no direct contact with pigs - from the Netherlands. They key messages being:

The Eurosurveillance report is lengthy and informative, and worth reading in its entirety. I've posted the link and some excerpts below. I'll have a bit more when you return.
Human infections with Eurasian avian-like swine influenza virus detected by coincidence via routine respiratory surveillance systems, the Netherlands, 2020 to 2023
Dirk Eggink1, Annelies Kroneman1 , Jozef Dingemans2 , Gabriel Goderski1 , Sharon van den Brink1 , Mariam Bagheri1 , Pascal Lexmond3 , Mark Pronk3 , Erhard van der Vries5 , Evelien Germeraad4 , Diederik Brandwagt1 , Manon Houben5 , Mariëtte van Hooiveld6 , Joke van der Giessen1 , Ron Fouchier3 , Adam Meijer1
Sporadic human infections with avian or swine influenza A virus (swIAV) have been reported. Zoonotic influenza, including human infections with avian influenza A virus or swIAV, is notifiable in the Netherlands and national and international guidelines state that local and national public health services need to be timely notified of laboratory-confirmed cases allowing source finding and contact tracing.
Swine influenza A viruses are genetically and antigenically distinct for different continents, due to introductions into pig populations from different origins and at different time points. Limited inter-continental spread between pigs or pig farms seems to occur [1-3]. In Europe, four swIAV haemagglutinin (HA) lineages are enzootic:
Swine influenza A viruses are genetically and antigenically distinct for different continents, due to introductions into pig populations from different origins and at different time points. Limited inter-continental spread between pigs or pig farms seems to occur [1-3]. In Europe, four swIAV haemagglutinin (HA) lineages are enzootic:
- H1 classical swine lineage (clade 1A) including H1pdm09-like viruses,
- H1 human seasonal lineage (clade 1B),
- H1 Eurasian avian-like lineage (clade 1C)
- and European human-like H3 lineage.
These HA lineages are combined with four neuraminidase (NA) lineages: N1pdm09-like, avian N1 and two swine N2 lineages. Knowledge about circulating genotypes of swIAV is limited and difficult to obtain due to limited surveillance of swIAV on pig farms in most countries in Europe, including the Netherlands, especially after the European Surveillance Network for Influenza in Pigs (ESNIP 3) was terminated in 2013 [1].
Human infections with swIAV have been detected sporadically in Europe whereas in the United States (US), such infections have been detected more frequently [2,4-6]. These infections occurred mainly after close contact with infected pigs on agricultural fairs [5]. In addition, the A(H1N1)pdm09 pandemic in 2009 was highly likely caused by direct spillover from infected pigs and subsequent human-to-human transmission [7].
Human infections with swIAV have been detected sporadically in Europe whereas in the United States (US), such infections have been detected more frequently [2,4-6]. These infections occurred mainly after close contact with infected pigs on agricultural fairs [5]. In addition, the A(H1N1)pdm09 pandemic in 2009 was highly likely caused by direct spillover from infected pigs and subsequent human-to-human transmission [7].
In the Netherlands, swIAV was detected in six persons 1986–2019 [4,8-13]. The previous detections of swIAV infections in humans in the Netherlands were mostly coincidental, in hospitalised persons presenting with severe disease [10], as no surveillance system to detect zoonotic spillovers from pigs to humans is in place with the aim to monitor specific risk groups upon exposure. This contrasts with monitoring of individuals exposed to poultry infected with highly pathogenic avian influenza A virus (HPAI) for which passive and active monitoring is in place in the Netherlands.
Here we describe detection of Eurasian swIAV infection in three persons during routine influenza surveillance activities in 2020, 2022 and 2023 in the Netherlands.
(SNIP)
Interestingly, the three patients described here were detected via routine surveillance systems and NIC activities. The occurrence of these infections outside the traditional respiratory season is remarkable, although it is not possible at this point to draw conclusions about the risk of infection during or outside the respiratory season.
In the Netherlands, there is no passive or active surveillance of humans exposed to pigs infected with swIAV, like farmers, their family members, veterinarians or individuals involved in transport or slaughtering of pigs. The experience in the US with swIAV exposure and human infections associated with visits to agricultural fairs suggests that close contact could be a major factor in zoonotic transmission, although the lack of direct exposure to (infected) pigs in two out of the three patients described, illustrates that other routes of transmission could exist.
Due to the possible cross-reactivity of anti A(H1N1)pdm09-like antibodies, present in most, if not all, humans, with the zoonotic viruses, serological assays could not be used for source and contact tracing to investigate possible unnoticed human-to-human transmission.
This likely results in underreporting of human infections with swIAV, in particular, as most patients with ILI in the general population are not sampled, let alone subtyped, especially those from mild to moderately severe cases that do not seek healthcare [28]. Therefore, (mild) human infections with swIAV could easily remain undetected.
Undetected infections might result in human adaptation by adaptive mutations or reassortment with seasonal influenza viruses, which could increase risk for human-to-human transmission.
In the Netherlands, there is no passive or active surveillance of humans exposed to pigs infected with swIAV, like farmers, their family members, veterinarians or individuals involved in transport or slaughtering of pigs. The experience in the US with swIAV exposure and human infections associated with visits to agricultural fairs suggests that close contact could be a major factor in zoonotic transmission, although the lack of direct exposure to (infected) pigs in two out of the three patients described, illustrates that other routes of transmission could exist.
Due to the possible cross-reactivity of anti A(H1N1)pdm09-like antibodies, present in most, if not all, humans, with the zoonotic viruses, serological assays could not be used for source and contact tracing to investigate possible unnoticed human-to-human transmission.
This likely results in underreporting of human infections with swIAV, in particular, as most patients with ILI in the general population are not sampled, let alone subtyped, especially those from mild to moderately severe cases that do not seek healthcare [28]. Therefore, (mild) human infections with swIAV could easily remain undetected.
Undetected infections might result in human adaptation by adaptive mutations or reassortment with seasonal influenza viruses, which could increase risk for human-to-human transmission.
(Continue . . . )
While avian flu currently gets the bulk of our attention due to the rapid evolution and spread of HPAI H5N1, we also keep close tabs on swine flu, because pigs are considered to be excellent reservoirs and `mixing vessels' for influenza.

While there are numerous swine-variants around the world, here in the United States the CDC has identified 3 as having some pandemic potential.
- H1N2 variant [A/California/62/2018] Jul 2019 5.8 5.7 Moderate
- H3N2 variant [A/Ohio/13/2017] Jul 2019 6.6 5.8 Moderate
- H3N2 variant [A/Indiana/08/2011] Dec 2012 6.0 4.5 Moderate
And of course, we now have increased concerns over the potential for avian H5N1 to spillover into pigs, as it already has with dairy cows. Thus far, only two pigs have tested positive in the United States, but elsewhere in the world we've seen sporadic reports of spillovers.
WHO H5N1 detected in pigs in China (2004)
EID Journal: Asymptomatic H5N1 In Pigs (2010)
An Unusual Report Of H5N1 in Pigs (Indonesia 2016)
Sci. Rpts.: Evidence Of H5N1 Exposure In Domestic Pigs - Nigeria (2018)
Seroconversion of a Swine Herd in a Free-Range Rural Multi-Species Farm against HPAI H5N1 2.3.4.4b Clade Virus (2023)
But the reality is, most of the world isn't even looking. Even here in the United States, testing of pigs for novel flu viruses is largely voluntary and anonymous. Since pigs are generally able to carry novel flu viruses (including H5N1) asymptomatically, passive surveillance is unlikely to detect infections.
While most of the emerging novel viruses we look at in this blog will probably never pose a genuine global health threat - there are a lot of contenders out there - and it only takes one overachiever to make an indelible mark on the world.
https://afludiary.blogspot.com/2025/...ions-with.html