Chikungunya virus disease- Global situation
3 October 2025
Situation at a glance
In 2025, a resurgence of chikungunya virus (CHIKV) disease was noted in a number of countries, including some that had not reported substantial case numbers in recent years. Between 1 January and 30 September 2025, a total of 445 271 suspected and confirmed CHIKV disease cases and 155 deaths were reported globally from 40 countries, including autochthonous and travel imported cases. Some WHO Regions are experiencing significant increases in case numbers compared to 2024, although others are currently reporting lower case numbers. This uneven distribution of cases across regions makes it challenging to characterize the situation as a global rise, however, given the ongoing outbreaks reported globally in 2025, the potential for further spread remains significant. CHIKV disease can be introduced into new areas by infected travelers and local transmission may be established if there is the presence of Aedes mosquito and a susceptible population. The risk is heightened by limited population immunity in previously unaffected areas, favorable environmental conditions for vector breeding, gaps in surveillance and diagnostic capacity, and increased human mobility and trade. Strengthening disease surveillance, enhancing vector surveillance and control, and improving public health preparedness are essential to mitigate the risk of further transmission. Prior to 2025, current or previous autochthonous transmission of CHIKV has been reported from 119 countries and territories. A total of 27 countries and territories across six WHO regions have established competent populations of Aedes aegypti mosquitoes but have not yet reported autochthonous CHIKV transmission. Additional countries have established populations of Aedes albopictus mosquitoes, which can also transmit CHIKV, and in which transmission efficiency is enhanced for CHIKV lineages with the E1 226V mutation. The presence of these vectors poses a continuous threat of chikungunya introduction and spread in previously unaffected areas. Increased CHIKV transmission is driven by multiple factors that include the expanded geographic distribution of Aedes mosquitoes related to transportation in conveyances and climate change, unplanned urbanization, poor water management, and weakened vector surveillance and control. CHIKV disease typically causes high population attack rates. In smaller settings such as islands, the transmission dynamics can be temporarily interrupted once a proportion of the population becomes infected and subsequently immune. In larger populations however, where enough individuals remain immunologically susceptible, transmission can persist over time, leading to sustained outbreaks. These outbreaks often place a significant burden on healthcare systems due to the number of affected individuals. Countries differ in their ability to detect and report chikungunya and other vector-borne diseases, with many outbreaks identified only retrospectively, hindering effective public health responses. Early detection of cases, particularly in persons at risk for severe CHIKV disease, and timely access to appropriate medical care are essential for minimizing clinical complications and reducing mortality. The variation in distribution of cases across regions highlights the importance of continued investment in surveillance, preparedness, and response capacities to address evolving regional dynamics. WHO continues to call on all countries to strengthen their healthcare and laboratory systems to enable rapid detection, timely reporting, and effective response to chikungunya outbreaks.
Description of the situation
Global overview
Globally as of December 2024, current or previous autochthonous transmission of CHIKV had been reported from 119 countries and territories across six WHO regions. In addition, 27 countries and territories had evidence of established and competent Aedes aegypti and Aedes albopictus vector populations but had not yet documented autochthonous CHIKV transmission.
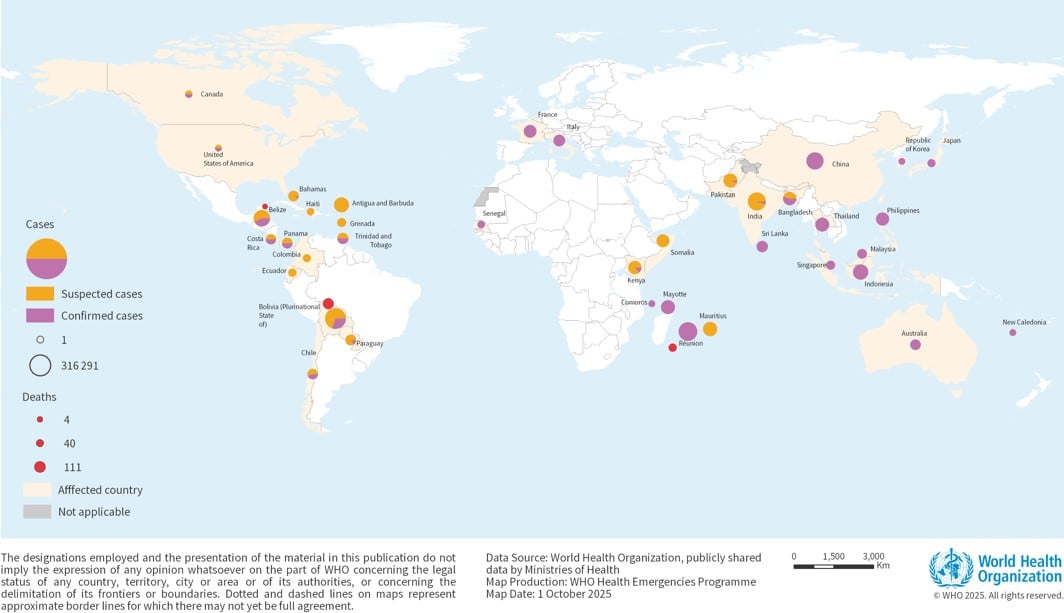
Per available data from January to September 2025, 263 592 suspected and 181 679 confirmed CHIKV disease cases and 155 CHIKV disease-related deaths have been reported globally. While certain WHO Regions are reporting lower case numbers compared to 2024, others are experiencing marked increases. This heterogeneity in regional trends complicates the interpretation of a global increase. Instead, the data suggest localized resurgence or emergence in specific geographic areas. The region of the Americas has reported the highest number of cases followed by the European region (comprised of cases reported predominantly from French Overseas Departments in the Indian Ocean).
Table 1: Number of suspected and confirmed CHIKV disease cases and deaths by region in 2025, as of September 2025.
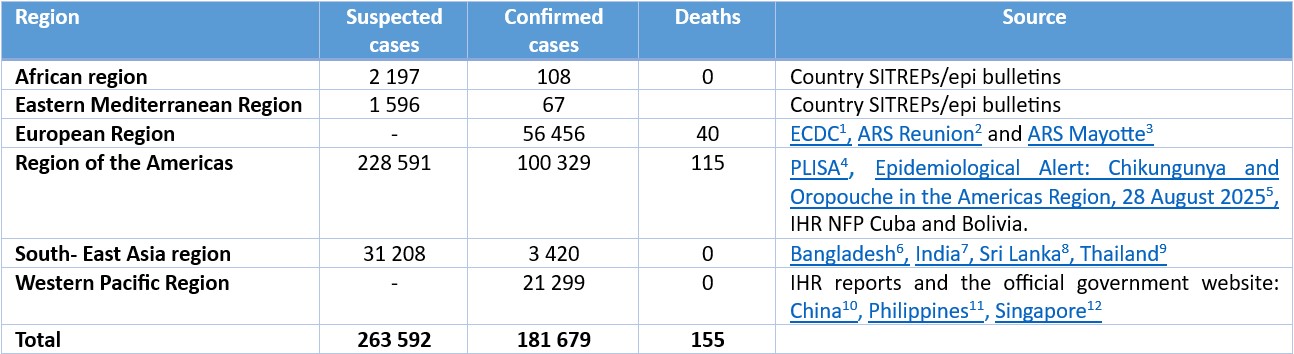
*Note: the date of last report varies by country
Figure 1: Geographical distribution of CHIKV diseasecases as reported to WHO or Publicly shared by Ministries of Health from January to September 2025
Regional overview
African region
As of September 2025, a total of 2197 suspected and 108 confirmed CHIKV disease cases have been reported from four countries: Comoros, Kenya, Mauritius, and Senegal with Mauritius recording the highest number of cases.
In Comoros, between 1 January to 31 May 2025 (epi week 1 and epi week 22), a total of four confirmed cases have been reported, while Senegal reported seven confirmed cases.
In Mauritius, a total of 1583 cases have been reported between 15 March to 4 August 2025 (epi week 12 to epi week 32), including 1543 local and 40 imported cases. There have been no reported deaths.
In Kenya, a chikungunya outbreak was confirmed in Mombasa County as of 8 June 2025 (epi week 23). By 6 July 2025 (epi week 27), a total of 614 cases had been recorded, including 97 laboratory-confirmed cases. Since then, no further cases have been reported. No chikungunya-related deaths have been recorded to date.
Eastern Mediterranean Region
As of September 2025, a total of 1596 suspected, and 67 confirmed CHIKV disease cases have been reported from Pakistan and Somalia.
In Pakistan, CHIKV disease cases in 2025 have been reported at rates similar to those in 2024. A notable increase occurred between 4 May to 21 June 2025 (epi weeks 19 to 25), with 101 to 121 suspected CHIKV disease cases reported per week during this period.
In Somalia, a chikungunya outbreak has been confirmed in Sool region, with 488 suspected cases reported between January and June 2025. Eight out of 10 samples tested were laboratory-confirmed for chikungunya. Somalia has also recorded imported travel related cases.
European Region
As of 15 September 2025, two European countries—France and Italy—have reported locally acquired cases of CHIKV disease. France has recorded 479 cases distributed across 54 clusters, with 40 clusters currently active. Italy has reported 205 locally acquired cases distributed across four clusters of which three clusters are currently active. A total of 56 456 CHIKV disease cases, and 40 deaths, have been reported from four countries in the European region in 2025.
In France, the increased incidence of CHIKV outbreaks in 2025 represent a deviation from observed patterns in previous years. In 2024, only one CHIKV disease case was reported. The larger number of chikungunya cases this year, along with their early onset, are linked to an epidemic in La Réunion and the broader Indian Ocean region, driven by a viral strain that is highly adapted to the Aedes albopictus mosquito.
In the French overseas department of La Réunion, a total of 54 517 confirmed cases and 40 deaths have been reported in 2025 (as of 14 September). There has been a steady decline in new cases since 26 April (epi week 17) indicating that the outbreak is waning. This marks the first autochthonous transmission of chikungunya on the island since 2014.
In Mayotte, following two imported cases from La Réunion, the first locally acquired CHIKV disease case was confirmed in March 2025. As of 18 September 2025, a total of 1255 locally acquired cases, including 39 hospitalizations, have been reported. The transmission receded since August with only a few cases reported per week on average.
Region of the Americas
As of 20 September 2025, CHIKV disease transmission continues across the Americas in line with expected seasonal patterns. A total of 228 591 suspected cases have been reported from 14 countries, including 100 329 confirmed cases and 115 deaths.
In Bolivia, a total of 5372 CHIKV disease cases have been reported, 73% of which are laboratory confirmed, along with four deaths. The outbreak primarily affected the department of Santa Cruz with 99% of cases (n=3905, including four deaths). Additionally, cases were reported in the departments of Beni, Chuquisaca, Cochabamba, Pando, and Tarija.
Brazil accounts for nearly 96% of all reported cases and deaths in the region, with 96 159 confirmed cases and 111 deaths.
In Cuba, between 1 January to 20 September, 34 cases of chikungunya were reported, all confirmed by laboratory by RT-PCR test, in the provinces of Guantanamo, La Habana, Matanzas, Pinar del Rio and Santiago de Cuba. Public health interventions have been implemented.
South- East Asia Region
As of early September 2025, over 34 628 CHIKV disease cases, both suspected and confirmed, have been reported in the WHO South-East Asia region, primarily from India and Bangladesh.
In India, between 1 January and 31 March 2025, a total of 30 876 suspected cases and 1741 confirmed cases were reported. The states reporting the highest number of confirmed cases were Maharashtra, Karnataka and Tamil Nadu.
In Bangladesh, the Institute of Epidemiology, Disease Control and Research between reported a total of 732 suspected CHIKV disease cases in Dhaka city between 1 January and 31 August 2025. Of these, 400 cases were laboratory-confirmed by RT-PCR.
In Sri Lanka, a total of 151 confirmed CHIKV disease cases were reported from sentinel sites in Colombo, Gampaha and Kandy between 1 January 2025 and the second week of March 2025. According to the Epidemiology Unit Division situation report, dated 31 August 2025, the CHIKV disease cases continued to increase and peaked in June 2025. Over half of the reported cases were from the Western Province, with Colombo District alone reporting 33%. The most affected age group was 41–60 years (36.4%), although an increasing trend was noted among children.
In Thailand, a total of 1128 CHIKV disease cases were reported between 1 January and 14 September 2025. Bueng Kan (142), Chiang Mai (411), and Loei (125) are the provinces reporting the most cases. The age distribution of cases is: 0-4 years 2%, 5-9 years 3%, 10-14 years 6%, 15-19 years 4%, 20-29 years 9%, 30-39 years 17%, 40-49 years 17%, 50-59 years 16%, ≥ 60 years 26%.
Western Pacific Region
A total of 14 359 CHIKV disease cases, with no deaths, have been reported from 16 countries and areas in the Western Pacific region in 2025. Of these, five countries reported local transmission, six reported imported cases, and five reported no cases during the year.
In China, as of 27 September 2025, a total of 16 452 locally transmitted cases has been reported in Guangdong Province. All cases were laboratory-confirmed. This represents the largest documented chikungunya outbreak to date in China. The cases have been reported in 21 cities, mainly in Foshan City (10032), Jiangmen City (5209), Guangzhou City (590), Shenzhen City (128), Zhanjiang City (112), Zhuhai City (60), and Zhongshan City (54). Additionally, during 1-21 September, Guangxi Zhuang Autonomous Region reported 297 local and associated cases; Fujian Province reported 124 local and associated cases; and some other provinces (such as Hunan, Sichuan, and Hainan provinces) also reported a few local cases.
According to the data as of 16 August 2025, among all locally reported cases nationwide, the age distribution is: 0-5 years 3.1 %, 6-17 years 13.6 %, 18-45 years 37.0 %, 46-60 years 23.3 %, 61-74 years 15.2 %, and ≥75 years 7.8 %. Up to now, all reported cases have been mild, with no severe cases or deaths.
In Indonesia, as of 31 July 2025, a total of 3608 confirmed CHIKV disease cases across 19 provinces have been reported, compared to 1399 confirmed cases reported during the same period in 2024. No chikungunya-related deaths have been recorded to date. The risk of future increases persists, particularly during the transition from the rainy to the dry season, with heightened concern in the most populous and frequently visited provinces: West Java, Central Java, East Java, and Banten. The Ministry of Health of Indonesia has strengthened detection and reporting through its Early Warning Alert and Response System (EWARS) and has implemented response measures in high-risk areas.
In Malaysia, as of 2 August 2025, a total of 40 CHIKV disease cases have been reported in 2025, compared to the 63 cases reported during the same period in 2024. No chikungunya-related deaths have been recorded to date. During the current reporting year, three chikungunya clusters were reported. Case investigation, integrated vector management, community engagement, and multisectoral collaboration efforts were implemented. All outbreaks were successfully contained within two weeks of detection, indicating an effective public health response and outbreak management.
In Philippines, as of 16 August 2025, a total of 628 CHIKV disease cases have been reported, a 78% decrease from 2886 cases reported in the same period in 2024. The national trend has been fluctuating, with 46 cases reported from 20 July to 2 August 2025, which is 10% lower than the 51 cases reported two weeks prior (6 to 19 July 2025). Cases ranged from 1 to 87 years old, with a median age of 33. Females accounted for 66% of cases (414 out of 628). There was one death reported (CFR: 0.16%). Local health authorities have investigated areas with clustering of cases to determine risk factors and implement vector control activities
In Singapore, as of 20 September 2025, 25 cases of CHIKV disease cases have been reported, compared to 12 cases reported during the same period in 2024. The majority of the cases were individuals with recent travel to chikungunya-affected areas. No chikungunya-related deaths and no sustained local transmission have been reported. The Communicable Diseases Agency Singapore continues to monitor the situation closely and provides ongoing guidance on prevention and control measures, particularly focusing on vector control to limit further transmission.
Epidemiology
Chikungunya is a mosquito-borne viral disease caused by the CHIKV, an RNA virus in the alphavirus genus of the family Togaviridae. CHIKV is transmitted by infected female mosquitoes, most commonly Aedes aegypti and Aedes albopictus, which can also transmit dengue and Zika viruses. These mosquitoes bite primarily during daylight hours and Aedes aegypti feeds both indoors and outdoors, whereas Aedes albopictus feeds primarily outdoors. They lay eggs in manmade and natural containers with standing water.
When an uninfected mosquito feeds on a person who has CHIKV circulating in their bloodstream, the mosquito can ingest the virus. Over a period of about 10 days (range: 7-12 days), the virus replicates in the mosquito and enters its salivary glands. Once this occurs, the mosquito becomes capable of transmitting the virus to a new human host through a subsequent bite. In the newly infected individual, the virus begins to replicate and reaches high concentrations in the blood, enabling further transmission to other mosquitos and perpetuating the transmission cycle.
In symptomatic patients, CHIKV disease onset is typically 4–8 days (range 2–12 days) after the bite of an infected mosquito. Disease is characterized by an abrupt onset of fever, frequently accompanied by severe joint pain. The joint pain is often debilitating and usually lasts for a few days but may be prolonged, lasting for weeks, months or even years. Other common signs and symptoms include joint swelling, muscle pain, headache, nausea, fatigue and rash. Since these symptoms overlap with other infections, including those with dengue and Zika viruses, cases can be misdiagnosed. In the absence of significant joint pain, symptoms in infected individuals are usually mild and the infection may go unrecognized. Most patients recover fully from the infection; however, occasional cases of eye, heart, and neurological complications have been reported with CHIKV infections. Patients at extremes of the age spectrum are at higher risk for severe disease including newborns infected during delivery to infected mothers or bitten by infected mosquitoes in the weeks after birth, and older people, particularly those with underlying medical conditions. Patients with severe disease require hospitalization because of the risk of organ damage and death. Once an individual is recovered, available evidence suggests they are likely to be immune from future chikungunya infections. CHIKV may be detected directly in blood samples collected during the first week of illness using molecular tests such as reverse transcriptase–polymerase chain reaction (RT–PCR), and after the first week of illness using serologic tests to detect antibodies produced in response to CHIKV infection.
Clinical management includes managing fever and joint pain with anti-pyretic, analgesics, maintaining adequate hydration by consuming sufficient fluids and ensuring general rest. There is no specific antiviral drug treatment for CHIKV infections. Paracetamol or acetaminophen are recommended for pain relief and reducing fever until dengue infections are ruled out, as non-steroidal anti-inflammatory drugs (NSAIDs) can increase the risk of bleeding.
There are currently two chikungunya vaccines that have received regulatory approvals and/or have been recommended for use in populations at risk in several countries, but the vaccines are not yet widely available nor in widespread use. WHO and external expert advisors are reviewing vaccine trial and post-marketing data in the context of global chikungunya epidemiology to inform possible recommendations for use.
Public health response
Overview of public health response in WHO Regions
WHO is supporting Member States in strengthening preparedness and response to arbovirus epidemics and pandemics, in alignment with the pillars of the Global Arbovirus Initiative, the Global Strategic Preparedness, Readiness and Response Plan, and the 5Cs of WHO’s global architecture for Strengthening health emergency prevention, preparedness, response and resilience (HEPR).
WHO has undertaken the following actions:
African region
In 2025, WHO and partners supported countries reporting chikungunya in the WHO African Region to respond to this outbreak. While sporadic transmission was reported in four countries, the most significant impact was observed in Mauritius and Kenya, where comprehensive response operations have been implemented.
Mauritius:
Kenya:
Eastern Mediterranean region
In response to the chikungunya outbreaks, WHO EMRO is taking the following actions:
European region
In response to the chikungunya situation in La Réunion and Mayotte, authorities activated the corresponding levels of the Organisation de la Réponse de Sécurité Civile (ORSEC) arbovirus plan.
Region of the Americas
PAHO has undertaken the following actions:
South-East Asia Region
WHO SEARO is supporting Member States by undertaking the following actions:
Western Pacific Region
In response to the chikungunya outbreaks, Member States have implemented response measures based on the evolving chikungunya situation at national and subnational levels:
CHIKV disease is an Aedes-borne disease widely distributed in tropical and subtropical regions. While the overall fatality rate is low, severe disease can occur, especially in vulnerable populations such as infants, the elderly, and those with pre-existing conditions. Chikungunya can be introduced to new areas by viremic travelers and generate local transmission in the presence of competent vectors, an immunologically susceptible population and favorable environmental conditions.
Factors associated with increased CHIKV transmission include:
The public health impact of the outbreaks depends on several factors, including the ability to detect chikungunya transmission, overall capacities for a coordinated public health response and clinical management, and the proportion of the population immunologically susceptible to CHIKV infection (i.e., people not previously infected with CHIKV). Young children, elderly individuals, and those with pre-existing health conditions such as diabetes, hypertension, and cardiovascular diseases are at higher risk of developing severe disease. Additionally, people living in areas with high mosquito populations and inadequate vector control measures are at greater risk of being infected. Effective public health strategies such as vector surveillance and control, and community education, are crucial in reducing the risk infection for susceptible individuals and preventing outbreaks.
In some areas, there is a lack of medical facilities with limited geographical access, making it difficult for people to access basic health care. Other challenges can be stockouts of several essential supplies for prevention and control, lack of reagents and consumables for laboratory diagnosis, and need for re-training field teams and health workers.
Given the ongoing outbreaks reported globally in 2025, the potential for further spread remains significant. Although some WHO Regions are currently reporting lower case numbers compared to 2024, others are experiencing a resurgence with significant increases in case numbers, furthermore some countries are seeing an emergence of chikungunya in previously unaffected populations. This uneven distribution of cases across regions makes it challenging to characterize the situation as a global rise but rather substantial increases in particular geographical areas. Further, the potential for geographic spread remains substantial given that chikungunya can be introduced into new areas by infected travelers where local transmission may be established in the presence of Aedes mosquito and a susceptible population. The risk is heightened by limited population immunity in previously unaffected areas, favorable environmental conditions for vector breeding, gaps in surveillance and diagnostic capacity, and increased human mobility and trade. Strengthening surveillance, enhancing vector surveillance and control, and improving public health preparedness are essential to mitigate the risk of further transmission.
WHO advice
WHO encourages countries to develop and maintain the capacity to detect and confirm cases, manage patients, and implement social communication strategies to gain community support to reduce the presence of the mosquito vectors. This includes training and alerting healthcare workers on case detection and potential complications, identifying risk groups for severe disease, ensuring appropriate clinical management, and following up on cases to prevent deaths. Targeted integrated vector surveillance and control measures are essential to reduce transmission rates.
Strengthening health care capacity:
Chikungunya can cause large outbreaks with high attack rates, affecting one-third to three-quarters of immunologically susceptible populations with the potential to cause heavy burdens on healthcare systems. Early detection of severe disease progression and access to proper medical attention are key to addressing clinical complications and reducing mortality, particularly among people at higher risk of severe disease (associated factors include age >65 years and <1 year, comorbidities, pregnant women specially in the third trimester due to vertical transmission during birth, which leads to neonatal chikungunya, with several complications such as encephalitis, hepatitis, and myocarditis).
Countries vary in their capacity to detect and report chikungunya and other vector-borne diseases, and outbreaks are often reported retrospectively, meaning real-time epidemiological data necessary for public health response is lacking. WHO reiterates to all countries the importance of strengthening their healthcare capacity to rapidly detect and report chikungunya and other vector-borne diseases to understand real-time epidemiological data necessary for public health response. It is important to include measures for appropriate clinical detection and management as well as preparing health services for surges in case counts.
Strengthening surveillance:
Enhanced surveillance and epidemiologic investigations are essential to more accurately determine the incidence and trends of CHIKV infection. Current surveillance systems often prioritize dengue over other Aedes-bornearboviruses, leading to frequent clinical misdiagnosis and misreporting of CHIKV as dengue. Strengthening surveillance capacity will support more effective risk communication and guide targeted vector control strategies. Ongoing, close monitoring of the regional situation is also critical, along with active cross-border coordination and information sharing, given the potential for transmission in neighboring countries.
Laboratory confirmation:
Although many countries have acquired the capacity to perform RT-PCR testing in recent years, the availability of reagents for chikungunya testing is limited and costs may be prohibitive. Serology is generally cheaper and more accessible but, in some regions, serological tests can cross-react with other alphaviruses and produce false positive test results.
Prevention and control:
Prevention efforts are highly focused on surveillance and control of predominantly day-biting Aedes mosquitoes. For protection against mosquito bites, clothing that minimizes skin exposure to mosquitoes indoors and outdoors is advised. Window and door screens should be used to prevent mosquitoes from entering homes. Repellents can be applied to exposed skin or clothing in strict accordance with product label instructions.
Avoidance of mosquito bites offers the best protection against CHIKV infection. Patients suspected of having CHIKV infection should avoid getting mosquito bites during the first week of illness to prevent further transmission to mosquitoes, that may in turn infect other people.
The main method to reduce transmission of CHIKV is through control of the mosquito vectors and reduction of mosquito breeding sites. This requires mobilization of communities, who are critical in reducing mosquito breeding sites through emptying and cleaning containers that contain water on a weekly basis, disposing of waste, and supporting local mosquito control programmes.
During outbreaks, intensified vector control activities targeting adult mosquitoes and larvae are a priority to interrupt the CHIKV transmission cycle. This may also be performed by health authorities as an emergency measure to control the mosquito population.
Insecticide-treated mosquito nets should be used against day-biting mosquitoes by persons who sleep during the daytime, for example young children, sick patients or older people.
No measures related to international traffic and trade are warranted at this time.
...
3 October 2025
Situation at a glance
In 2025, a resurgence of chikungunya virus (CHIKV) disease was noted in a number of countries, including some that had not reported substantial case numbers in recent years. Between 1 January and 30 September 2025, a total of 445 271 suspected and confirmed CHIKV disease cases and 155 deaths were reported globally from 40 countries, including autochthonous and travel imported cases. Some WHO Regions are experiencing significant increases in case numbers compared to 2024, although others are currently reporting lower case numbers. This uneven distribution of cases across regions makes it challenging to characterize the situation as a global rise, however, given the ongoing outbreaks reported globally in 2025, the potential for further spread remains significant. CHIKV disease can be introduced into new areas by infected travelers and local transmission may be established if there is the presence of Aedes mosquito and a susceptible population. The risk is heightened by limited population immunity in previously unaffected areas, favorable environmental conditions for vector breeding, gaps in surveillance and diagnostic capacity, and increased human mobility and trade. Strengthening disease surveillance, enhancing vector surveillance and control, and improving public health preparedness are essential to mitigate the risk of further transmission. Prior to 2025, current or previous autochthonous transmission of CHIKV has been reported from 119 countries and territories. A total of 27 countries and territories across six WHO regions have established competent populations of Aedes aegypti mosquitoes but have not yet reported autochthonous CHIKV transmission. Additional countries have established populations of Aedes albopictus mosquitoes, which can also transmit CHIKV, and in which transmission efficiency is enhanced for CHIKV lineages with the E1 226V mutation. The presence of these vectors poses a continuous threat of chikungunya introduction and spread in previously unaffected areas. Increased CHIKV transmission is driven by multiple factors that include the expanded geographic distribution of Aedes mosquitoes related to transportation in conveyances and climate change, unplanned urbanization, poor water management, and weakened vector surveillance and control. CHIKV disease typically causes high population attack rates. In smaller settings such as islands, the transmission dynamics can be temporarily interrupted once a proportion of the population becomes infected and subsequently immune. In larger populations however, where enough individuals remain immunologically susceptible, transmission can persist over time, leading to sustained outbreaks. These outbreaks often place a significant burden on healthcare systems due to the number of affected individuals. Countries differ in their ability to detect and report chikungunya and other vector-borne diseases, with many outbreaks identified only retrospectively, hindering effective public health responses. Early detection of cases, particularly in persons at risk for severe CHIKV disease, and timely access to appropriate medical care are essential for minimizing clinical complications and reducing mortality. The variation in distribution of cases across regions highlights the importance of continued investment in surveillance, preparedness, and response capacities to address evolving regional dynamics. WHO continues to call on all countries to strengthen their healthcare and laboratory systems to enable rapid detection, timely reporting, and effective response to chikungunya outbreaks.
Description of the situation
Global overview
Globally as of December 2024, current or previous autochthonous transmission of CHIKV had been reported from 119 countries and territories across six WHO regions. In addition, 27 countries and territories had evidence of established and competent Aedes aegypti and Aedes albopictus vector populations but had not yet documented autochthonous CHIKV transmission.
Per available data from January to September 2025, 263 592 suspected and 181 679 confirmed CHIKV disease cases and 155 CHIKV disease-related deaths have been reported globally. While certain WHO Regions are reporting lower case numbers compared to 2024, others are experiencing marked increases. This heterogeneity in regional trends complicates the interpretation of a global increase. Instead, the data suggest localized resurgence or emergence in specific geographic areas. The region of the Americas has reported the highest number of cases followed by the European region (comprised of cases reported predominantly from French Overseas Departments in the Indian Ocean).
Table 1: Number of suspected and confirmed CHIKV disease cases and deaths by region in 2025, as of September 2025.

*Note: the date of last report varies by country
Figure 1: Geographical distribution of CHIKV diseasecases as reported to WHO or Publicly shared by Ministries of Health from January to September 2025

Regional overview
African region
As of September 2025, a total of 2197 suspected and 108 confirmed CHIKV disease cases have been reported from four countries: Comoros, Kenya, Mauritius, and Senegal with Mauritius recording the highest number of cases.
In Comoros, between 1 January to 31 May 2025 (epi week 1 and epi week 22), a total of four confirmed cases have been reported, while Senegal reported seven confirmed cases.
In Mauritius, a total of 1583 cases have been reported between 15 March to 4 August 2025 (epi week 12 to epi week 32), including 1543 local and 40 imported cases. There have been no reported deaths.
In Kenya, a chikungunya outbreak was confirmed in Mombasa County as of 8 June 2025 (epi week 23). By 6 July 2025 (epi week 27), a total of 614 cases had been recorded, including 97 laboratory-confirmed cases. Since then, no further cases have been reported. No chikungunya-related deaths have been recorded to date.
Eastern Mediterranean Region
As of September 2025, a total of 1596 suspected, and 67 confirmed CHIKV disease cases have been reported from Pakistan and Somalia.
In Pakistan, CHIKV disease cases in 2025 have been reported at rates similar to those in 2024. A notable increase occurred between 4 May to 21 June 2025 (epi weeks 19 to 25), with 101 to 121 suspected CHIKV disease cases reported per week during this period.
In Somalia, a chikungunya outbreak has been confirmed in Sool region, with 488 suspected cases reported between January and June 2025. Eight out of 10 samples tested were laboratory-confirmed for chikungunya. Somalia has also recorded imported travel related cases.
European Region
As of 15 September 2025, two European countries—France and Italy—have reported locally acquired cases of CHIKV disease. France has recorded 479 cases distributed across 54 clusters, with 40 clusters currently active. Italy has reported 205 locally acquired cases distributed across four clusters of which three clusters are currently active. A total of 56 456 CHIKV disease cases, and 40 deaths, have been reported from four countries in the European region in 2025.
In France, the increased incidence of CHIKV outbreaks in 2025 represent a deviation from observed patterns in previous years. In 2024, only one CHIKV disease case was reported. The larger number of chikungunya cases this year, along with their early onset, are linked to an epidemic in La Réunion and the broader Indian Ocean region, driven by a viral strain that is highly adapted to the Aedes albopictus mosquito.
In the French overseas department of La Réunion, a total of 54 517 confirmed cases and 40 deaths have been reported in 2025 (as of 14 September). There has been a steady decline in new cases since 26 April (epi week 17) indicating that the outbreak is waning. This marks the first autochthonous transmission of chikungunya on the island since 2014.
In Mayotte, following two imported cases from La Réunion, the first locally acquired CHIKV disease case was confirmed in March 2025. As of 18 September 2025, a total of 1255 locally acquired cases, including 39 hospitalizations, have been reported. The transmission receded since August with only a few cases reported per week on average.
Region of the Americas
As of 20 September 2025, CHIKV disease transmission continues across the Americas in line with expected seasonal patterns. A total of 228 591 suspected cases have been reported from 14 countries, including 100 329 confirmed cases and 115 deaths.
In Bolivia, a total of 5372 CHIKV disease cases have been reported, 73% of which are laboratory confirmed, along with four deaths. The outbreak primarily affected the department of Santa Cruz with 99% of cases (n=3905, including four deaths). Additionally, cases were reported in the departments of Beni, Chuquisaca, Cochabamba, Pando, and Tarija.
Brazil accounts for nearly 96% of all reported cases and deaths in the region, with 96 159 confirmed cases and 111 deaths.
In Cuba, between 1 January to 20 September, 34 cases of chikungunya were reported, all confirmed by laboratory by RT-PCR test, in the provinces of Guantanamo, La Habana, Matanzas, Pinar del Rio and Santiago de Cuba. Public health interventions have been implemented.
South- East Asia Region
As of early September 2025, over 34 628 CHIKV disease cases, both suspected and confirmed, have been reported in the WHO South-East Asia region, primarily from India and Bangladesh.
In India, between 1 January and 31 March 2025, a total of 30 876 suspected cases and 1741 confirmed cases were reported. The states reporting the highest number of confirmed cases were Maharashtra, Karnataka and Tamil Nadu.
In Bangladesh, the Institute of Epidemiology, Disease Control and Research between reported a total of 732 suspected CHIKV disease cases in Dhaka city between 1 January and 31 August 2025. Of these, 400 cases were laboratory-confirmed by RT-PCR.
In Sri Lanka, a total of 151 confirmed CHIKV disease cases were reported from sentinel sites in Colombo, Gampaha and Kandy between 1 January 2025 and the second week of March 2025. According to the Epidemiology Unit Division situation report, dated 31 August 2025, the CHIKV disease cases continued to increase and peaked in June 2025. Over half of the reported cases were from the Western Province, with Colombo District alone reporting 33%. The most affected age group was 41–60 years (36.4%), although an increasing trend was noted among children.
In Thailand, a total of 1128 CHIKV disease cases were reported between 1 January and 14 September 2025. Bueng Kan (142), Chiang Mai (411), and Loei (125) are the provinces reporting the most cases. The age distribution of cases is: 0-4 years 2%, 5-9 years 3%, 10-14 years 6%, 15-19 years 4%, 20-29 years 9%, 30-39 years 17%, 40-49 years 17%, 50-59 years 16%, ≥ 60 years 26%.
Western Pacific Region
A total of 14 359 CHIKV disease cases, with no deaths, have been reported from 16 countries and areas in the Western Pacific region in 2025. Of these, five countries reported local transmission, six reported imported cases, and five reported no cases during the year.
In China, as of 27 September 2025, a total of 16 452 locally transmitted cases has been reported in Guangdong Province. All cases were laboratory-confirmed. This represents the largest documented chikungunya outbreak to date in China. The cases have been reported in 21 cities, mainly in Foshan City (10032), Jiangmen City (5209), Guangzhou City (590), Shenzhen City (128), Zhanjiang City (112), Zhuhai City (60), and Zhongshan City (54). Additionally, during 1-21 September, Guangxi Zhuang Autonomous Region reported 297 local and associated cases; Fujian Province reported 124 local and associated cases; and some other provinces (such as Hunan, Sichuan, and Hainan provinces) also reported a few local cases.
According to the data as of 16 August 2025, among all locally reported cases nationwide, the age distribution is: 0-5 years 3.1 %, 6-17 years 13.6 %, 18-45 years 37.0 %, 46-60 years 23.3 %, 61-74 years 15.2 %, and ≥75 years 7.8 %. Up to now, all reported cases have been mild, with no severe cases or deaths.
In Indonesia, as of 31 July 2025, a total of 3608 confirmed CHIKV disease cases across 19 provinces have been reported, compared to 1399 confirmed cases reported during the same period in 2024. No chikungunya-related deaths have been recorded to date. The risk of future increases persists, particularly during the transition from the rainy to the dry season, with heightened concern in the most populous and frequently visited provinces: West Java, Central Java, East Java, and Banten. The Ministry of Health of Indonesia has strengthened detection and reporting through its Early Warning Alert and Response System (EWARS) and has implemented response measures in high-risk areas.
In Malaysia, as of 2 August 2025, a total of 40 CHIKV disease cases have been reported in 2025, compared to the 63 cases reported during the same period in 2024. No chikungunya-related deaths have been recorded to date. During the current reporting year, three chikungunya clusters were reported. Case investigation, integrated vector management, community engagement, and multisectoral collaboration efforts were implemented. All outbreaks were successfully contained within two weeks of detection, indicating an effective public health response and outbreak management.
In Philippines, as of 16 August 2025, a total of 628 CHIKV disease cases have been reported, a 78% decrease from 2886 cases reported in the same period in 2024. The national trend has been fluctuating, with 46 cases reported from 20 July to 2 August 2025, which is 10% lower than the 51 cases reported two weeks prior (6 to 19 July 2025). Cases ranged from 1 to 87 years old, with a median age of 33. Females accounted for 66% of cases (414 out of 628). There was one death reported (CFR: 0.16%). Local health authorities have investigated areas with clustering of cases to determine risk factors and implement vector control activities
In Singapore, as of 20 September 2025, 25 cases of CHIKV disease cases have been reported, compared to 12 cases reported during the same period in 2024. The majority of the cases were individuals with recent travel to chikungunya-affected areas. No chikungunya-related deaths and no sustained local transmission have been reported. The Communicable Diseases Agency Singapore continues to monitor the situation closely and provides ongoing guidance on prevention and control measures, particularly focusing on vector control to limit further transmission.
Epidemiology
Chikungunya is a mosquito-borne viral disease caused by the CHIKV, an RNA virus in the alphavirus genus of the family Togaviridae. CHIKV is transmitted by infected female mosquitoes, most commonly Aedes aegypti and Aedes albopictus, which can also transmit dengue and Zika viruses. These mosquitoes bite primarily during daylight hours and Aedes aegypti feeds both indoors and outdoors, whereas Aedes albopictus feeds primarily outdoors. They lay eggs in manmade and natural containers with standing water.
When an uninfected mosquito feeds on a person who has CHIKV circulating in their bloodstream, the mosquito can ingest the virus. Over a period of about 10 days (range: 7-12 days), the virus replicates in the mosquito and enters its salivary glands. Once this occurs, the mosquito becomes capable of transmitting the virus to a new human host through a subsequent bite. In the newly infected individual, the virus begins to replicate and reaches high concentrations in the blood, enabling further transmission to other mosquitos and perpetuating the transmission cycle.
In symptomatic patients, CHIKV disease onset is typically 4–8 days (range 2–12 days) after the bite of an infected mosquito. Disease is characterized by an abrupt onset of fever, frequently accompanied by severe joint pain. The joint pain is often debilitating and usually lasts for a few days but may be prolonged, lasting for weeks, months or even years. Other common signs and symptoms include joint swelling, muscle pain, headache, nausea, fatigue and rash. Since these symptoms overlap with other infections, including those with dengue and Zika viruses, cases can be misdiagnosed. In the absence of significant joint pain, symptoms in infected individuals are usually mild and the infection may go unrecognized. Most patients recover fully from the infection; however, occasional cases of eye, heart, and neurological complications have been reported with CHIKV infections. Patients at extremes of the age spectrum are at higher risk for severe disease including newborns infected during delivery to infected mothers or bitten by infected mosquitoes in the weeks after birth, and older people, particularly those with underlying medical conditions. Patients with severe disease require hospitalization because of the risk of organ damage and death. Once an individual is recovered, available evidence suggests they are likely to be immune from future chikungunya infections. CHIKV may be detected directly in blood samples collected during the first week of illness using molecular tests such as reverse transcriptase–polymerase chain reaction (RT–PCR), and after the first week of illness using serologic tests to detect antibodies produced in response to CHIKV infection.
Clinical management includes managing fever and joint pain with anti-pyretic, analgesics, maintaining adequate hydration by consuming sufficient fluids and ensuring general rest. There is no specific antiviral drug treatment for CHIKV infections. Paracetamol or acetaminophen are recommended for pain relief and reducing fever until dengue infections are ruled out, as non-steroidal anti-inflammatory drugs (NSAIDs) can increase the risk of bleeding.
There are currently two chikungunya vaccines that have received regulatory approvals and/or have been recommended for use in populations at risk in several countries, but the vaccines are not yet widely available nor in widespread use. WHO and external expert advisors are reviewing vaccine trial and post-marketing data in the context of global chikungunya epidemiology to inform possible recommendations for use.
Public health response
Overview of public health response in WHO Regions
WHO is supporting Member States in strengthening preparedness and response to arbovirus epidemics and pandemics, in alignment with the pillars of the Global Arbovirus Initiative, the Global Strategic Preparedness, Readiness and Response Plan, and the 5Cs of WHO’s global architecture for Strengthening health emergency prevention, preparedness, response and resilience (HEPR).
WHO has undertaken the following actions:
- Issued an alert at the UN Geneva Press Briefing in July 2025 regarding the rapid global spread of chikungunya.
African region
In 2025, WHO and partners supported countries reporting chikungunya in the WHO African Region to respond to this outbreak. While sporadic transmission was reported in four countries, the most significant impact was observed in Mauritius and Kenya, where comprehensive response operations have been implemented.
Mauritius:
- Surveillance has been strengthened to detect new cases early and monitor transmission trends.
- Vector control measures have been implemented including larval source reduction, household spraying, and fogging.
- Community engagement activities have been implemented through awareness campaigns promoting personal protection, household vector control, and early care-seeking.
- A Rapid Risk Assessment was conducted with WHO support to guide response actions.
Kenya:
- A national response plan was developed, and an Incident Management System team was established for coordination.
- The Ministry of Health issued advisories to coastal counties, including Mombasa, to reinforce surveillance and clinical case management.
- Vector control activities were implemented including treating 2198 mosquito breeding sites with larvicide, fogging in 306 villages, and spraying 218 houses.
- Community sensitization activities were conducted which reached 780 people, and insecticide-treated nets were distributed to vulnerable groups.
Eastern Mediterranean region
In response to the chikungunya outbreaks, WHO EMRO is taking the following actions:
- A webinar to provide an orientation for national health authorities, public health professionals, and relevant stakeholders, offering a comprehensive overview of chikungunya epidemiology and outbreak prevention and control is planned.
- An online training session for Somalia on case management was conducted.
- Technical support is being provided to review national multisectoral arboviral response plans in affected countries, ensuring alignment with WHO-recommended strategies, integrated vector management, case management protocols, laboratory preparedness, and risk communication approaches.
European region
In response to the chikungunya situation in La Réunion and Mayotte, authorities activated the corresponding levels of the Organisation de la Réponse de Sécurité Civile (ORSEC) arbovirus plan.
- Enhanced surveillance, began on 1 May 2025, is being carried out at the local level (traps, individual reporting by citizens, etc.). This surveillance should enable the detection of new mosquito species. It is accompanied by health interventions around detected cases, whether imported or indigenous, particularly during periods of enhanced surveillance.
- Door-to-door surveys are being carried out for enhanced entomological and epidemiological investigations and active search for clinically suggestive cases;
- Vector control treatments in the affected area are being carried out (adulticide mosquito control treatments within a 300-metre radius of the case) ;
- Awareness is being increased among healthcare professionals in the sector and local authorities;
- The Ministry of Health published several press releases specifying the actions planned by the health authorities and best practices for vector control for the general population, and regularly posts updates on its social media accounts. Safety measures for products of human origin are implemented in accordance with the recommendations of the High Council for Public Health, including a 28-day deferral for donors of labile blood products and the introduction of viral genomic diagnosis.
- Posters and prevention messages targeting travellers are currently displayed on aircraft (for flights between mainland France and Réunion, as well as to the French West Indies and French Guiana) and in airport terminals to raise awareness.
- In Italy, in the regions affected by local transmission events, all the control measures set out in the National Plan for the Prevention, Surveillance and Response to Arboviral Diseases have been implemented, including the immediate activation of epidemiological and entomological surveillance, the adoption of vector control interventions, population protection measures, and the safeguarding of transfusion and transplant safety.
Region of the Americas
PAHO has undertaken the following actions:
- Issued an Epidemiological alert on chikungunya in the Americas Region on 28 August 2025 regarding the rapid global spread of chikungunya.
- Provides advice and technical support to prevent and control chikungunya on the basis of the Integrated Management Strategy for Arboviral Disease Prevention and Control, adopted by PAHO/WHO Member States in 2016 (CD55.R6).
- Support is being provided for the implementation of an integrated epidemiological surveillance system model for dengue, chikungunya and Zika. This model integrates epidemiological, clinical, laboratory and entomological surveillance to generate standardized and timely information for decision-making.
- Technical support is being provided to implement a collaborative surveillance strategy for arboviral diseases, operationalized through virtual collaboration spaces (VCS). Through the VCS, countries and PAHO collaborate in the real-time analysis of their epidemiological, clinical, laboratory and entomological data, as well as the generation of automated reports and bulletins.
- National capacities are being strengthened for clinical diagnosis and case management of dengue, chikungunya, and Zika in the Region through the implementation of technical documents and clinical guidelines, virtual continuous training strategy that includes distance-based self-learning courses, and the establishment of national networks of clinical experts in arboviral diseases. Developed several initiatives to improve entomological surveillance systems, as well as the monitoring and management of resistance to insecticides used in public health.
- In August 2025, a workshop was held on updating the Integrated Management Strategy for the Prevention and Control of Arboviral Diseases (IMS-Arbovirus) and the roadmap for the implementation, monitoring, and evaluation of the IMS-Arbovirus in countries and territories of the Americas for the period 2026–2030.
- Public health laboratories are being supported in the implementation of RT-PCR testing and serology (IgM) interpretation (technical cooperation, training, provision of key reagents) and of genomic surveillance for chikungunya monitoring and characterization.
- Entomo-virological surveillance guidelines are currently being implemented to enable early detection and assessment.
- A new operational model for Aedes control is being developed and is currently in the implementation phase in the Americas Region.
- Vector-control activities are being strengthened in affected countries.
South-East Asia Region
WHO SEARO is supporting Member States by undertaking the following actions:
- Continuing to monitor the situation and raise awareness of chikungunya and other arboviruses in the region.
- Enhancing vector control for Aedes mosquitos to better control chikungunya and other arbovirus, strengthening community engagement.
- Advocacy and planning for integrated arbovirus surveillance—covering dengue, chikungunya, and Zika—are underway to generate stronger evidence for informed decision-making.
- The implementation of guidelines on the clinical management of chikungunya is actively being promoted.
Western Pacific Region
- The WHO Regional Office for the Western Pacific has implemented the following measures:Shared the global situation and technical guidelines with Member States in the Western Pacific Region to support chikungunya preparedness and response. These include: (1) WHO Chikungunya Outbreak Toolbox, (2) WHO Guidelines for Clinical Management of Arboviral Diseases (Dengue, Chikungunya, Zika, and Yellow Fever) – 4 July 2025, and (3) Policy Considerations for Strengthening Preparedness and Response to Arbovirus Epidemics and Pandemics – 16 July 2025
- Requested Member States in the WPR to provide updates on the chikungunya situation in their respective countries for risk assessment.
- Conducted a Community of Practice (CoP) session on 14 August 2025 to share updates and best practices with Member States.
In response to the chikungunya outbreaks, Member States have implemented response measures based on the evolving chikungunya situation at national and subnational levels:
- National and local health authorities have been conducting field investigations in areas with case clustering and outbreaks to assess transmission dynamics, inform decision-making, and coordinate multisectoral response efforts in the affected areas.
- Medical care is being provided to affected individuals, including hospitalization or home isolation for laboratory-confirmed cases, ensuring appropriate clinical management and reducing transmission risk.
- Surveillance systems have been strengthened to enable early detection, laboratory confirmation, and timely reporting of cases. Enhanced monitoring in healthcare facilities is supporting rapid identification and response to potential clusters and importation risks.
- Targeted vector control interventions are being implemented in high-risk areas, including environmental cleanup to eliminate breeding sites, larviciding, and adult mosquito control through fogging operations. Innovative approaches such as drone-assisted spraying are also being utilized.
- Community health workers are mobilized for door-to-door inspections and public education campaigns to promote source reduction and personal protection. Authorities are maintaining regular communication with the public to share updates, promote preventive measures, and address concerns.
CHIKV disease is an Aedes-borne disease widely distributed in tropical and subtropical regions. While the overall fatality rate is low, severe disease can occur, especially in vulnerable populations such as infants, the elderly, and those with pre-existing conditions. Chikungunya can be introduced to new areas by viremic travelers and generate local transmission in the presence of competent vectors, an immunologically susceptible population and favorable environmental conditions.
Factors associated with increased CHIKV transmission include:
- broader geographic range of Aedes mosquitoes associated with transport of immature stages in goods and vessels, and expansion of conducive mosquito habitats due to climate change and periodic extreme events, with increased potential for chikungunya transmission in previously unaffected areas;
- unplanned urbanization and poor water management contribute to vector reproduction and persistent transmission cycles;
- low coverage or lack of sustainability of the vector control programs.
- political instability and conflict in countries at risk, such as Somalia, Sudan, and Yemen face exacerbated public health challenges include disruption of healthcare infrastructure hampering case detection and outbreak response;
- increased travel to and from endemic regions introducing cases into areas with established populations of competent mosquito vectors and favorable environmental conditions.
The public health impact of the outbreaks depends on several factors, including the ability to detect chikungunya transmission, overall capacities for a coordinated public health response and clinical management, and the proportion of the population immunologically susceptible to CHIKV infection (i.e., people not previously infected with CHIKV). Young children, elderly individuals, and those with pre-existing health conditions such as diabetes, hypertension, and cardiovascular diseases are at higher risk of developing severe disease. Additionally, people living in areas with high mosquito populations and inadequate vector control measures are at greater risk of being infected. Effective public health strategies such as vector surveillance and control, and community education, are crucial in reducing the risk infection for susceptible individuals and preventing outbreaks.
In some areas, there is a lack of medical facilities with limited geographical access, making it difficult for people to access basic health care. Other challenges can be stockouts of several essential supplies for prevention and control, lack of reagents and consumables for laboratory diagnosis, and need for re-training field teams and health workers.
Given the ongoing outbreaks reported globally in 2025, the potential for further spread remains significant. Although some WHO Regions are currently reporting lower case numbers compared to 2024, others are experiencing a resurgence with significant increases in case numbers, furthermore some countries are seeing an emergence of chikungunya in previously unaffected populations. This uneven distribution of cases across regions makes it challenging to characterize the situation as a global rise but rather substantial increases in particular geographical areas. Further, the potential for geographic spread remains substantial given that chikungunya can be introduced into new areas by infected travelers where local transmission may be established in the presence of Aedes mosquito and a susceptible population. The risk is heightened by limited population immunity in previously unaffected areas, favorable environmental conditions for vector breeding, gaps in surveillance and diagnostic capacity, and increased human mobility and trade. Strengthening surveillance, enhancing vector surveillance and control, and improving public health preparedness are essential to mitigate the risk of further transmission.
WHO advice
WHO encourages countries to develop and maintain the capacity to detect and confirm cases, manage patients, and implement social communication strategies to gain community support to reduce the presence of the mosquito vectors. This includes training and alerting healthcare workers on case detection and potential complications, identifying risk groups for severe disease, ensuring appropriate clinical management, and following up on cases to prevent deaths. Targeted integrated vector surveillance and control measures are essential to reduce transmission rates.
Strengthening health care capacity:
Chikungunya can cause large outbreaks with high attack rates, affecting one-third to three-quarters of immunologically susceptible populations with the potential to cause heavy burdens on healthcare systems. Early detection of severe disease progression and access to proper medical attention are key to addressing clinical complications and reducing mortality, particularly among people at higher risk of severe disease (associated factors include age >65 years and <1 year, comorbidities, pregnant women specially in the third trimester due to vertical transmission during birth, which leads to neonatal chikungunya, with several complications such as encephalitis, hepatitis, and myocarditis).
Countries vary in their capacity to detect and report chikungunya and other vector-borne diseases, and outbreaks are often reported retrospectively, meaning real-time epidemiological data necessary for public health response is lacking. WHO reiterates to all countries the importance of strengthening their healthcare capacity to rapidly detect and report chikungunya and other vector-borne diseases to understand real-time epidemiological data necessary for public health response. It is important to include measures for appropriate clinical detection and management as well as preparing health services for surges in case counts.
Strengthening surveillance:
Enhanced surveillance and epidemiologic investigations are essential to more accurately determine the incidence and trends of CHIKV infection. Current surveillance systems often prioritize dengue over other Aedes-bornearboviruses, leading to frequent clinical misdiagnosis and misreporting of CHIKV as dengue. Strengthening surveillance capacity will support more effective risk communication and guide targeted vector control strategies. Ongoing, close monitoring of the regional situation is also critical, along with active cross-border coordination and information sharing, given the potential for transmission in neighboring countries.
Laboratory confirmation:
Although many countries have acquired the capacity to perform RT-PCR testing in recent years, the availability of reagents for chikungunya testing is limited and costs may be prohibitive. Serology is generally cheaper and more accessible but, in some regions, serological tests can cross-react with other alphaviruses and produce false positive test results.
Prevention and control:
Prevention efforts are highly focused on surveillance and control of predominantly day-biting Aedes mosquitoes. For protection against mosquito bites, clothing that minimizes skin exposure to mosquitoes indoors and outdoors is advised. Window and door screens should be used to prevent mosquitoes from entering homes. Repellents can be applied to exposed skin or clothing in strict accordance with product label instructions.
Avoidance of mosquito bites offers the best protection against CHIKV infection. Patients suspected of having CHIKV infection should avoid getting mosquito bites during the first week of illness to prevent further transmission to mosquitoes, that may in turn infect other people.
The main method to reduce transmission of CHIKV is through control of the mosquito vectors and reduction of mosquito breeding sites. This requires mobilization of communities, who are critical in reducing mosquito breeding sites through emptying and cleaning containers that contain water on a weekly basis, disposing of waste, and supporting local mosquito control programmes.
During outbreaks, intensified vector control activities targeting adult mosquitoes and larvae are a priority to interrupt the CHIKV transmission cycle. This may also be performed by health authorities as an emergency measure to control the mosquito population.
Insecticide-treated mosquito nets should be used against day-biting mosquitoes by persons who sleep during the daytime, for example young children, sick patients or older people.
No measures related to international traffic and trade are warranted at this time.
...
