Re: Seasonal Flu 2008 - 2009
Commentary at
Commentary at
 </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD align=middle width=780 bgColor=#0063c8 height=1>EISS - Weekly Electronic Bulletin </TD><TD width=1 bgColor=black height=1>
</TD><TD align=middle width=780 bgColor=#0063c8 height=1>EISS - Weekly Electronic Bulletin </TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#0063c8 height=1><TABLE border=0><TBODY><TR bgColor=white><TD noWrap width=254 bgColor=#0063c8>Week 42 : 13/10/2008-19/10/2008</TD><TD noWrap align=middle width=258 bgColor=#0063c8></TD><TD noWrap align=right width=254 bgColor=#0063c8>24 October 2008, Issue N? 276</TD></TR></TBODY></TABLE></TD><TD width=1 bgColor=black height=1>
</TD><TD width=780 bgColor=#0063c8 height=1><TABLE border=0><TBODY><TR bgColor=white><TD noWrap width=254 bgColor=#0063c8>Week 42 : 13/10/2008-19/10/2008</TD><TD noWrap align=middle width=258 bgColor=#0063c8></TD><TD noWrap align=right width=254 bgColor=#0063c8>24 October 2008, Issue N? 276</TD></TR></TBODY></TABLE></TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=3>
</TD><TD width=780 bgColor=#1d92ff height=3> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>
</TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>
 </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE width=780 border=0><TBODY><TR><TD>Map
</TD><TD width=780 bgColor=white><TABLE width=780 border=0><TBODY><TR><TD>Map </CENTER>
</CENTER> </TD><TD vAlign=top>Low = no influenza activity or influenza at baseline levels
</TD><TD vAlign=top>Low = no influenza activity or influenza at baseline levels </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>Network comments (where available)
</TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>Network comments (where available) </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE width=780><TBODY><TR><TD>Table and graphs (where available)
</TD><TD width=780 bgColor=white><TABLE width=780><TBODY><TR><TD>Table and graphs (where available) </TD></TR><TR><TD vAlign=top bgColor=white></TD><TD vAlign=top bgColor=white>Intensity</TD><TD vAlign=top bgColor=white>Geographic
</TD></TR><TR><TD vAlign=top bgColor=white></TD><TD vAlign=top bgColor=white>Intensity</TD><TD vAlign=top bgColor=white>Geographic </TD></TR><TR id=r2c0><TD class=relative id=r2c1 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Belgium</TD><TD class=relative id=r2c2 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Low</TD><TD class=relative id=r2c3 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>None</TD><TD class=relative id=r2c4 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>8</TD><TD class=relative id=r2c5 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>0%</TD><TD class=relative id=r2c6 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) noWrap>None</TD><TD class=relative id=r2c7 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>42.2</TD><TD class=relative id=r2c8 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c9 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>1467.7</TD><TD class=relative id=r2c10 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c11 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Bulgaria</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>641.3 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r3c0><TD class=relative id=r3c1 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Czech Republic</TD><TD class=relative id=r3c2 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Low</TD><TD class=relative id=r3c3 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>None</TD><TD class=relative id=r3c4 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>18</TD><TD class=relative id=r3c5 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>0%</TD><TD class=relative id=r3c6 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) noWrap>None</TD><TD class=relative id=r3c7 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>20.6 </TD><TD class=relative id=r3c8 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c9 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>922.6 </TD><TD class=relative id=r3c10 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c11 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Click here</TD><TR id=r4c0><TD class=relative id=r4c1 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Denmark</TD><TD class=relative id=r4c2 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)></TD><TD class=relative id=r4c3 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)></TD><TD class=relative id=r4c4 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>0</TD><TD class=relative id=r4c5 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>0%</TD><TD class=relative id=r4c6 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) noWrap>None</TD><TD class=relative id=r4c7 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) align=right></TD><TD class=relative id=r4c8 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>(graphs)</TD><TD class=relative id=r4c9 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) noWrap></TD><TD class=relative id=r4c10 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)></TD><TD class=relative id=r4c11 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Click here</TD><TR id=r5c0><TD class=relative id=r5c1 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>England</TD><TD class=relative id=r5c2 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Low</TD><TD class=relative id=r5c3 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Sporadic</TD><TD class=relative id=r5c4 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>37</TD><TD class=relative id=r5c5 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>0%</TD><TD class=relative id=r5c6 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) noWrap>Type A</TD><TD class=relative id=r5c7 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>6.9 </TD><TD class=relative id=r5c8 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c9 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>556.8 </TD><TD class=relative id=r5c10 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c11 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Click here</TD><TR id=r6c0><TD class=relative id=r6c1 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Estonia</TD><TD class=relative id=r6c2 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Low</TD><TD class=relative id=r6c3 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>None</TD><TD class=relative id=r6c4 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>5</TD><TD class=relative id=r6c5 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>0%</TD><TD class=relative id=r6c6 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) noWrap>None</TD><TD class=relative id=r6c7 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>0.5</TD><TD class=relative id=r6c8 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c9 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>297.1</TD><TD class=relative id=r6c10 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c11 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Germany</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>14</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>776.0 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r9c0><TD class=relative id=r9c1 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Greece</TD><TD class=relative id=r9c2 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Low</TD><TD class=relative id=r9c3 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>None</TD><TD class=relative id=r9c4 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>0</TD><TD class=relative id=r9c5 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>0%</TD><TD class=relative id=r9c6 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) noWrap>None</TD><TD class=relative id=r9c7 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) align=right>0.0</TD><TD class=relative id=r9c8 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>(graphs)</TD><TD class=relative id=r9c9 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) align=right>64.0</TD><TD class=relative id=r9c10 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>(graphs)</TD><TD class=relative id=r9c11 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Click here</TD><TR id=r10c0><TD class=relative id=r10c1 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Hungary</TD><TD class=relative id=r10c2 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Low</TD><TD class=relative id=r10c3 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>None</TD><TD class=relative id=r10c4 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>3</TD><TD class=relative id=r10c5 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>0%</TD><TD class=relative id=r10c6 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) noWrap>None</TD><TD class=relative id=r10c7 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>115.1</TD><TD class=relative id=r10c8 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c9 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>0.0</TD><TD class=relative id=r10c10 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c11 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Click here</TD><TR id=r11c0><TD class=relative id=r11c1 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Ireland</TD><TD class=relative id=r11c2 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Low</TD><TD class=relative id=r11c3 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Sporadic</TD><TD class=relative id=r11c4 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>3</TD><TD class=relative id=r11c5 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>66.7%</TD><TD class=relative id=r11c6 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) noWrap>Type A</TD><TD class=relative id=r11c7 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>7.3 </TD><TD class=relative id=r11c8 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c9 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>0.0 </TD><TD class=relative id=r11c10 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c11 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Click here</TD><TR id=r13c0><TD class=relative id=r13c1 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Latvia</TD><TD class=relative id=r13c2 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Low</TD><TD class=relative id=r13c3 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>None</TD><TD class=relative id=r13c4 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0</TD><TD class=relative id=r13c5 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0%</TD><TD class=relative id=r13c6 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) noWrap>None</TD><TD class=relative id=r13c7 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>0.0 </TD><TD class=relative id=r13c8 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c9 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>1009.1 </TD><TD class=relative id=r13c10 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c11 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Click here</TD><TR id=r14c0><TD class=relative id=r14c1 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Lithuania</TD><TD class=relative id=r14c2 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Low</TD><TD class=relative id=r14c3 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>None</TD><TD class=relative id=r14c4 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0</TD><TD class=relative id=r14c5 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0%</TD><TD class=relative id=r14c6 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) noWrap>None</TD><TD class=relative id=r14c7 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>0.4 </TD><TD class=relative id=r14c8 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c9 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>484.4 </TD><TD class=relative id=r14c10 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c11 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Click here</TD><TR id=r17c0><TD class=relative id=r17c1 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Netherlands</TD><TD class=relative id=r17c2 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Low</TD><TD class=relative id=r17c3 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>None</TD><TD class=relative id=r17c4 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>7</TD><TD class=relative id=r17c5 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>14.3%</TD><TD class=relative id=r17c6 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) noWrap>None</TD><TD class=relative id=r17c7 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>28.0 </TD><TD class=relative id=r17c8 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c9 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>0.0 </TD><TD class=relative id=r17c10 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c11 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Click here</TD><TR id=r18c0><TD class=relative id=r18c1 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Northern Ireland</TD><TD class=relative id=r18c2 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)></TD><TD class=relative id=r18c3 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)></TD><TD class=relative id=r18c4 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>1</TD><TD class=relative id=r18c5 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>0%</TD><TD class=relative id=r18c6 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) noWrap>None</TD><TD class=relative id=r18c7 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) align=right></TD><TD class=relative id=r18c8 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>(graphs)</TD><TD class=relative id=r18c9 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) noWrap></TD><TD class=relative id=r18c10 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)></TD><TD class=relative id=r18c11 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Click here</TD><TR id=r19c0><TD class=relative id=r19c1 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Norway</TD><TD class=relative id=r19c2 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Low</TD><TD class=relative id=r19c3 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>None</TD><TD class=relative id=r19c4 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>2</TD><TD class=relative id=r19c5 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>0%</TD><TD class=relative id=r19c6 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) noWrap>None</TD><TD class=relative id=r19c7 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>23.0 </TD><TD class=relative id=r19c8 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c9 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>0.0 </TD><TD class=relative id=r19c10 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c11 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Click here</TD><TR id=r20c0><TD class=relative id=r20c1 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Poland</TD><TD class=relative id=r20c2 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Low</TD><TD class=relative id=r20c3 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>None</TD><TD class=relative id=r20c4 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>10</TD><TD class=relative id=r20c5 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>0%</TD><TD class=relative id=r20c6 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) noWrap>None</TD><TD class=relative id=r20c7 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>33.8 </TD><TD class=relative id=r20c8 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c9 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>0.0 </TD><TD class=relative id=r20c10 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c11 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Click here</TD><TR id=r21c0><TD class=relative id=r21c1 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Portugal</TD><TD class=relative id=r21c2 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Low</TD><TD class=relative id=r21c3 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>None</TD><TD class=relative id=r21c4 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>4</TD><TD class=relative id=r21c5 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>0%</TD><TD class=relative id=r21c6 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) noWrap>None</TD><TD class=relative id=r21c7 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>9.2 </TD><TD class=relative id=r21c8 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c9 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>0.0 </TD><TD class=relative id=r21c10 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c11 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Click here</TD><TR id=r22c0><TD class=relative id=r22c1 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Romania</TD><TD class=relative id=r22c2 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Low</TD><TD class=relative id=r22c3 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>None</TD><TD class=relative id=r22c4 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>7</TD><TD class=relative id=r22c5 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>0%</TD><TD class=relative id=r22c6 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) noWrap>None</TD><TD class=relative id=r22c7 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>0.0 </TD><TD class=relative id=r22c8 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c9 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>944.1 </TD><TD class=relative id=r22c10 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c11 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Click here</TD><TR id=r24c0><TD class=relative id=r24c1 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Serbia</TD><TD class=relative id=r24c2 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Low</TD><TD class=relative id=r24c3 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>None</TD><TD class=relative id=r24c4 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0</TD><TD class=relative id=r24c5 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0%</TD><TD class=relative id=r24c6 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) noWrap>None</TD><TD class=relative id=r24c7 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>73.8</TD><TD class=relative id=r24c8 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c9 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>0.0</TD><TD class=relative id=r24c10 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c11 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Click here</TD><TR id=r25c0><TD class=relative id=r25c1 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Slovakia</TD><TD class=relative id=r25c2 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Low</TD><TD class=relative id=r25c3 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>None</TD><TD class=relative id=r25c4 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>2</TD><TD class=relative id=r25c5 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>0%</TD><TD class=relative id=r25c6 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) noWrap>None</TD><TD class=relative id=r25c7 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>171.5 </TD><TD class=relative id=r25c8 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c9 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>1584.4 </TD><TD class=relative id=r25c10 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c11 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Click here</TD><TR id=r26c0><TD class=relative id=r26c1 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Slovenia</TD><TD class=relative id=r26c2 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Low</TD><TD class=relative id=r26c3 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>None</TD><TD class=relative id=r26c4 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>3</TD><TD class=relative id=r26c5 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>0%</TD><TD class=relative id=r26c6 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) noWrap>None</TD><TD class=relative id=r26c7 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>0.0 </TD><TD class=relative id=r26c8 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c9 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>775.3 </TD><TD class=relative id=r26c10 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c11 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Click here</TD><TR id=r27c0><TD class=relative id=r27c1 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Spain</TD><TD class=relative id=r27c2 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Low</TD><TD class=relative id=r27c3 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>None</TD><TD class=relative id=r27c4 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>30</TD><TD class=relative id=r27c5 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>0%</TD><TD class=relative id=r27c6 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) noWrap>None</TD><TD class=relative id=r27c7 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>14.1 </TD><TD class=relative id=r27c8 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c9 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>0.0 </TD><TD class=relative id=r27c10 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c11 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Click here</TD><TR id=r28c0><TD class=relative id=r28c1 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Sweden</TD><TD class=relative id=r28c2 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Low</TD><TD class=relative id=r28c3 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>None</TD><TD class=relative id=r28c4 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>43</TD><TD class=relative id=r28c5 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>2.3%</TD><TD class=relative id=r28c6 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) noWrap>Type A</TD><TD class=relative id=r28c7 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>1.3 </TD><TD class=relative id=r28c8 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c9 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>0.0 </TD><TD class=relative id=r28c10 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c11 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Click here</TD><TR id=r29c0><TD class=relative id=r29c1 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>Switzerland</TD><TD class=relative id=r29c2 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>Low</TD><TD class=relative id=r29c3 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>None</TD><TD class=relative id=r29c4 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>2</TD><TD class=relative id=r29c5 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>0%</TD><TD class=relative id=r29c6 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11) noWrap>None</TD><TD class=relative id=r29c7 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11) align=right>9.0 </TD><TD class=relative id=r29c8 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>(graphs)</TD><TD class=relative id=r29c9 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11) noWrap></TD><TD class=relative id=r29c10 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)></TD><TD class=relative id=r29c11 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>Click here</TD><TR id=r30c0><TD class=relative id=r30c1 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>Wales</TD><TD class=relative id=r30c2 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>Low</TD><TD class=relative id=r30c3 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>None</TD><TD class=relative id=r30c4 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c5 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c6 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11) noWrap></TD><TD class=relative id=r30c7 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11) align=right>1.0 </TD><TD class=relative id=r30c8 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c9 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11) align=right>0.0 </TD><TD class=relative id=r30c10 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c11 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>Click here</TD><TR id=r31c0><TD class=relative id=r31c1 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>Europe</TD><TD class=relative id=r31c2 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c3 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c4 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>199</TD><TD class=relative id=r31c5 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>2.0%</TD><TD class=relative id=r31c6 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c7 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11) align=right></TD><TD class=relative id=r31c8 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c9 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c10 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c11 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>Click here</TD><TR><TD bgColor=#808080 colSpan=14>
</TD></TR><TR id=r2c0><TD class=relative id=r2c1 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Belgium</TD><TD class=relative id=r2c2 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Low</TD><TD class=relative id=r2c3 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>None</TD><TD class=relative id=r2c4 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>8</TD><TD class=relative id=r2c5 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>0%</TD><TD class=relative id=r2c6 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) noWrap>None</TD><TD class=relative id=r2c7 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>42.2</TD><TD class=relative id=r2c8 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c9 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>1467.7</TD><TD class=relative id=r2c10 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c11 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Bulgaria</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>641.3 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r3c0><TD class=relative id=r3c1 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Czech Republic</TD><TD class=relative id=r3c2 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Low</TD><TD class=relative id=r3c3 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>None</TD><TD class=relative id=r3c4 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>18</TD><TD class=relative id=r3c5 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>0%</TD><TD class=relative id=r3c6 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) noWrap>None</TD><TD class=relative id=r3c7 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>20.6 </TD><TD class=relative id=r3c8 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c9 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>922.6 </TD><TD class=relative id=r3c10 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c11 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Click here</TD><TR id=r4c0><TD class=relative id=r4c1 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Denmark</TD><TD class=relative id=r4c2 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)></TD><TD class=relative id=r4c3 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)></TD><TD class=relative id=r4c4 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>0</TD><TD class=relative id=r4c5 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>0%</TD><TD class=relative id=r4c6 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) noWrap>None</TD><TD class=relative id=r4c7 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) align=right></TD><TD class=relative id=r4c8 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>(graphs)</TD><TD class=relative id=r4c9 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) noWrap></TD><TD class=relative id=r4c10 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)></TD><TD class=relative id=r4c11 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Click here</TD><TR id=r5c0><TD class=relative id=r5c1 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>England</TD><TD class=relative id=r5c2 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Low</TD><TD class=relative id=r5c3 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Sporadic</TD><TD class=relative id=r5c4 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>37</TD><TD class=relative id=r5c5 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>0%</TD><TD class=relative id=r5c6 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) noWrap>Type A</TD><TD class=relative id=r5c7 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>6.9 </TD><TD class=relative id=r5c8 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c9 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>556.8 </TD><TD class=relative id=r5c10 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c11 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Click here</TD><TR id=r6c0><TD class=relative id=r6c1 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Estonia</TD><TD class=relative id=r6c2 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Low</TD><TD class=relative id=r6c3 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>None</TD><TD class=relative id=r6c4 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>5</TD><TD class=relative id=r6c5 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>0%</TD><TD class=relative id=r6c6 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) noWrap>None</TD><TD class=relative id=r6c7 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>0.5</TD><TD class=relative id=r6c8 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c9 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>297.1</TD><TD class=relative id=r6c10 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c11 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Germany</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>14</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>776.0 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r9c0><TD class=relative id=r9c1 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Greece</TD><TD class=relative id=r9c2 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Low</TD><TD class=relative id=r9c3 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>None</TD><TD class=relative id=r9c4 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>0</TD><TD class=relative id=r9c5 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>0%</TD><TD class=relative id=r9c6 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) noWrap>None</TD><TD class=relative id=r9c7 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) align=right>0.0</TD><TD class=relative id=r9c8 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>(graphs)</TD><TD class=relative id=r9c9 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) align=right>64.0</TD><TD class=relative id=r9c10 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>(graphs)</TD><TD class=relative id=r9c11 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Click here</TD><TR id=r10c0><TD class=relative id=r10c1 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Hungary</TD><TD class=relative id=r10c2 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Low</TD><TD class=relative id=r10c3 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>None</TD><TD class=relative id=r10c4 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>3</TD><TD class=relative id=r10c5 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>0%</TD><TD class=relative id=r10c6 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) noWrap>None</TD><TD class=relative id=r10c7 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>115.1</TD><TD class=relative id=r10c8 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c9 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>0.0</TD><TD class=relative id=r10c10 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c11 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Click here</TD><TR id=r11c0><TD class=relative id=r11c1 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Ireland</TD><TD class=relative id=r11c2 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Low</TD><TD class=relative id=r11c3 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Sporadic</TD><TD class=relative id=r11c4 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>3</TD><TD class=relative id=r11c5 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>66.7%</TD><TD class=relative id=r11c6 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) noWrap>Type A</TD><TD class=relative id=r11c7 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>7.3 </TD><TD class=relative id=r11c8 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c9 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>0.0 </TD><TD class=relative id=r11c10 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c11 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Click here</TD><TR id=r13c0><TD class=relative id=r13c1 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Latvia</TD><TD class=relative id=r13c2 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Low</TD><TD class=relative id=r13c3 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>None</TD><TD class=relative id=r13c4 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0</TD><TD class=relative id=r13c5 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0%</TD><TD class=relative id=r13c6 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) noWrap>None</TD><TD class=relative id=r13c7 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>0.0 </TD><TD class=relative id=r13c8 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c9 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>1009.1 </TD><TD class=relative id=r13c10 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c11 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Click here</TD><TR id=r14c0><TD class=relative id=r14c1 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Lithuania</TD><TD class=relative id=r14c2 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Low</TD><TD class=relative id=r14c3 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>None</TD><TD class=relative id=r14c4 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0</TD><TD class=relative id=r14c5 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0%</TD><TD class=relative id=r14c6 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) noWrap>None</TD><TD class=relative id=r14c7 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>0.4 </TD><TD class=relative id=r14c8 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c9 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>484.4 </TD><TD class=relative id=r14c10 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c11 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Click here</TD><TR id=r17c0><TD class=relative id=r17c1 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Netherlands</TD><TD class=relative id=r17c2 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Low</TD><TD class=relative id=r17c3 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>None</TD><TD class=relative id=r17c4 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>7</TD><TD class=relative id=r17c5 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>14.3%</TD><TD class=relative id=r17c6 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) noWrap>None</TD><TD class=relative id=r17c7 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>28.0 </TD><TD class=relative id=r17c8 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c9 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>0.0 </TD><TD class=relative id=r17c10 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c11 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Click here</TD><TR id=r18c0><TD class=relative id=r18c1 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Northern Ireland</TD><TD class=relative id=r18c2 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)></TD><TD class=relative id=r18c3 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)></TD><TD class=relative id=r18c4 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>1</TD><TD class=relative id=r18c5 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>0%</TD><TD class=relative id=r18c6 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) noWrap>None</TD><TD class=relative id=r18c7 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) align=right></TD><TD class=relative id=r18c8 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>(graphs)</TD><TD class=relative id=r18c9 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) noWrap></TD><TD class=relative id=r18c10 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)></TD><TD class=relative id=r18c11 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Click here</TD><TR id=r19c0><TD class=relative id=r19c1 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Norway</TD><TD class=relative id=r19c2 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Low</TD><TD class=relative id=r19c3 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>None</TD><TD class=relative id=r19c4 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>2</TD><TD class=relative id=r19c5 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>0%</TD><TD class=relative id=r19c6 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) noWrap>None</TD><TD class=relative id=r19c7 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>23.0 </TD><TD class=relative id=r19c8 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c9 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>0.0 </TD><TD class=relative id=r19c10 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c11 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Click here</TD><TR id=r20c0><TD class=relative id=r20c1 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Poland</TD><TD class=relative id=r20c2 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Low</TD><TD class=relative id=r20c3 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>None</TD><TD class=relative id=r20c4 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>10</TD><TD class=relative id=r20c5 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>0%</TD><TD class=relative id=r20c6 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) noWrap>None</TD><TD class=relative id=r20c7 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>33.8 </TD><TD class=relative id=r20c8 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c9 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>0.0 </TD><TD class=relative id=r20c10 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c11 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Click here</TD><TR id=r21c0><TD class=relative id=r21c1 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Portugal</TD><TD class=relative id=r21c2 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Low</TD><TD class=relative id=r21c3 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>None</TD><TD class=relative id=r21c4 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>4</TD><TD class=relative id=r21c5 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>0%</TD><TD class=relative id=r21c6 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) noWrap>None</TD><TD class=relative id=r21c7 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>9.2 </TD><TD class=relative id=r21c8 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c9 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>0.0 </TD><TD class=relative id=r21c10 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c11 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Click here</TD><TR id=r22c0><TD class=relative id=r22c1 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Romania</TD><TD class=relative id=r22c2 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Low</TD><TD class=relative id=r22c3 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>None</TD><TD class=relative id=r22c4 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>7</TD><TD class=relative id=r22c5 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>0%</TD><TD class=relative id=r22c6 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) noWrap>None</TD><TD class=relative id=r22c7 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>0.0 </TD><TD class=relative id=r22c8 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c9 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>944.1 </TD><TD class=relative id=r22c10 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c11 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Click here</TD><TR id=r24c0><TD class=relative id=r24c1 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Serbia</TD><TD class=relative id=r24c2 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Low</TD><TD class=relative id=r24c3 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>None</TD><TD class=relative id=r24c4 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0</TD><TD class=relative id=r24c5 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0%</TD><TD class=relative id=r24c6 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) noWrap>None</TD><TD class=relative id=r24c7 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>73.8</TD><TD class=relative id=r24c8 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c9 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>0.0</TD><TD class=relative id=r24c10 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c11 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Click here</TD><TR id=r25c0><TD class=relative id=r25c1 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Slovakia</TD><TD class=relative id=r25c2 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Low</TD><TD class=relative id=r25c3 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>None</TD><TD class=relative id=r25c4 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>2</TD><TD class=relative id=r25c5 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>0%</TD><TD class=relative id=r25c6 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) noWrap>None</TD><TD class=relative id=r25c7 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>171.5 </TD><TD class=relative id=r25c8 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c9 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>1584.4 </TD><TD class=relative id=r25c10 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c11 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Click here</TD><TR id=r26c0><TD class=relative id=r26c1 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Slovenia</TD><TD class=relative id=r26c2 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Low</TD><TD class=relative id=r26c3 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>None</TD><TD class=relative id=r26c4 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>3</TD><TD class=relative id=r26c5 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>0%</TD><TD class=relative id=r26c6 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) noWrap>None</TD><TD class=relative id=r26c7 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>0.0 </TD><TD class=relative id=r26c8 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c9 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>775.3 </TD><TD class=relative id=r26c10 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c11 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Click here</TD><TR id=r27c0><TD class=relative id=r27c1 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Spain</TD><TD class=relative id=r27c2 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Low</TD><TD class=relative id=r27c3 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>None</TD><TD class=relative id=r27c4 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>30</TD><TD class=relative id=r27c5 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>0%</TD><TD class=relative id=r27c6 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) noWrap>None</TD><TD class=relative id=r27c7 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>14.1 </TD><TD class=relative id=r27c8 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c9 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>0.0 </TD><TD class=relative id=r27c10 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c11 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Click here</TD><TR id=r28c0><TD class=relative id=r28c1 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Sweden</TD><TD class=relative id=r28c2 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Low</TD><TD class=relative id=r28c3 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>None</TD><TD class=relative id=r28c4 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>43</TD><TD class=relative id=r28c5 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>2.3%</TD><TD class=relative id=r28c6 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) noWrap>Type A</TD><TD class=relative id=r28c7 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>1.3 </TD><TD class=relative id=r28c8 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c9 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>0.0 </TD><TD class=relative id=r28c10 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c11 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Click here</TD><TR id=r29c0><TD class=relative id=r29c1 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>Switzerland</TD><TD class=relative id=r29c2 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>Low</TD><TD class=relative id=r29c3 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>None</TD><TD class=relative id=r29c4 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>2</TD><TD class=relative id=r29c5 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>0%</TD><TD class=relative id=r29c6 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11) noWrap>None</TD><TD class=relative id=r29c7 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11) align=right>9.0 </TD><TD class=relative id=r29c8 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>(graphs)</TD><TD class=relative id=r29c9 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11) noWrap></TD><TD class=relative id=r29c10 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)></TD><TD class=relative id=r29c11 onmouseover=crossRow_over(29,11) onmouseout=crossRow_out(29,11)>Click here</TD><TR id=r30c0><TD class=relative id=r30c1 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>Wales</TD><TD class=relative id=r30c2 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>Low</TD><TD class=relative id=r30c3 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>None</TD><TD class=relative id=r30c4 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c5 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c6 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11) noWrap></TD><TD class=relative id=r30c7 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11) align=right>1.0 </TD><TD class=relative id=r30c8 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c9 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11) align=right>0.0 </TD><TD class=relative id=r30c10 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c11 onmouseover=crossRow_over(30,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(30,11)>Click here</TD><TR id=r31c0><TD class=relative id=r31c1 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>Europe</TD><TD class=relative id=r31c2 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c3 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c4 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>199</TD><TD class=relative id=r31c5 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>2.0%</TD><TD class=relative id=r31c6 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c7 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11) align=right></TD><TD class=relative id=r31c8 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c9 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c10 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c11 onmouseover=crossRow_over(31,11) style="BACKGROUND: #ffffff; CURSOR: hand" onmouseout=crossRow_out(31,11)>Click here</TD><TR><TD bgColor=#808080 colSpan=14> </TD></TR><TR><TD colSpan=14>Preliminary data</TD><TR><TD colSpan=14>
</TD></TR><TR><TD colSpan=14>Preliminary data</TD><TR><TD colSpan=14> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD><TABLE><TBODY><TR><TD>The bulletin text was written by an editorial team at the European Centre for Disease Prevention and Control (ECDC) and the Community Network of Reference Laboratories for Human Influenza in Europe (CNRL). Team members are Flaviu Plata, Phillip Zucs and Bruno Ciancio from ECDC, and Adam, Meijer Rod Daniels Alan Hay and Maria Zambon from CNRL. The bulletin text was reviewed by Olav Hungnes (Norwegian Institute of Public Health, Oslo, Norway), and Anne Mazick (Statens Serum Institut, Copenhagen, Denmark) on behalf of the EISS members.
</TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD><TABLE><TBODY><TR><TD>The bulletin text was written by an editorial team at the European Centre for Disease Prevention and Control (ECDC) and the Community Network of Reference Laboratories for Human Influenza in Europe (CNRL). Team members are Flaviu Plata, Phillip Zucs and Bruno Ciancio from ECDC, and Adam, Meijer Rod Daniels Alan Hay and Maria Zambon from CNRL. The bulletin text was reviewed by Olav Hungnes (Norwegian Institute of Public Health, Oslo, Norway), and Anne Mazick (Statens Serum Institut, Copenhagen, Denmark) on behalf of the EISS members. </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD align=middle width=780 bgColor=#0063c8>EISS : Weekly Electronic Bulletin</TD></TR></TBODY></TABLE>
</TD><TD align=middle width=780 bgColor=#0063c8>EISS : Weekly Electronic Bulletin</TD></TR></TBODY></TABLE>
 </TD></TR></TBODY></TABLE>
</TD></TR></TBODY></TABLE>
 </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#0063c8 height=1><TABLE border=0><TBODY><TR bgColor=white><TD noWrap width=254 bgColor=#0063c8>Week 43 : 20/10/2008-26/10/2008</TD><TD noWrap align=middle width=258 bgColor=#0063c8></TD><TD noWrap align=right width=254 bgColor=#0063c8>31 October 2008, Issue N? 277</TD></TR></TBODY></TABLE></TD><TD width=1 bgColor=black height=1>
</TD><TD width=780 bgColor=#0063c8 height=1><TABLE border=0><TBODY><TR bgColor=white><TD noWrap width=254 bgColor=#0063c8>Week 43 : 20/10/2008-26/10/2008</TD><TD noWrap align=middle width=258 bgColor=#0063c8></TD><TD noWrap align=right width=254 bgColor=#0063c8>31 October 2008, Issue N? 277</TD></TR></TBODY></TABLE></TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=3>
</TD><TD width=780 bgColor=#1d92ff height=3> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>
</TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>
 </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE width=780 border=0><TBODY><TR><TD>Map
</TD><TD width=780 bgColor=white><TABLE width=780 border=0><TBODY><TR><TD>Map </CENTER>
</CENTER> </TD><TD vAlign=top>Low = no influenza activity or influenza at baseline levels
</TD><TD vAlign=top>Low = no influenza activity or influenza at baseline levels </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>Network comments (where available)
</TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD>Network comments (where available) </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE width=780><TBODY><TR><TD>Table and graphs (where available)
</TD><TD width=780 bgColor=white><TABLE width=780><TBODY><TR><TD>Table and graphs (where available) </TD></TR><TR><TD vAlign=top bgColor=white></TD><TD vAlign=top bgColor=white>Intensity</TD><TD vAlign=top bgColor=white>Geographic
</TD></TR><TR><TD vAlign=top bgColor=white></TD><TD vAlign=top bgColor=white>Intensity</TD><TD vAlign=top bgColor=white>Geographic </TD></TR><TR id=r2c0><TD class=relative id=r2c1 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Belgium</TD><TD class=relative id=r2c2 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Low</TD><TD class=relative id=r2c3 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>None</TD><TD class=relative id=r2c4 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>15</TD><TD class=relative id=r2c5 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>0%</TD><TD class=relative id=r2c6 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) noWrap>None</TD><TD class=relative id=r2c7 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>38.6</TD><TD class=relative id=r2c8 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c9 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>270.3</TD><TD class=relative id=r2c10 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c11 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Bulgaria</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>589.0 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r3c0><TD class=relative id=r3c1 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Czech Republic</TD><TD class=relative id=r3c2 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Low</TD><TD class=relative id=r3c3 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>None</TD><TD class=relative id=r3c4 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>20</TD><TD class=relative id=r3c5 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>0%</TD><TD class=relative id=r3c6 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) noWrap>None</TD><TD class=relative id=r3c7 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>22.6 </TD><TD class=relative id=r3c8 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c9 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>935.7 </TD><TD class=relative id=r3c10 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c11 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Click here</TD><TR id=r4c0><TD class=relative id=r4c1 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Denmark</TD><TD class=relative id=r4c2 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Low</TD><TD class=relative id=r4c3 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>None</TD><TD class=relative id=r4c4 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>2</TD><TD class=relative id=r4c5 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>0%</TD><TD class=relative id=r4c6 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) noWrap>None</TD><TD class=relative id=r4c7 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) align=right>9.9 </TD><TD class=relative id=r4c8 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>(graphs)</TD><TD class=relative id=r4c9 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) align=right>0.0 </TD><TD class=relative id=r4c10 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>(graphs)</TD><TD class=relative id=r4c11 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Click here</TD><TR id=r5c0><TD class=relative id=r5c1 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>England</TD><TD class=relative id=r5c2 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Low</TD><TD class=relative id=r5c3 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Sporadic</TD><TD class=relative id=r5c4 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>35</TD><TD class=relative id=r5c5 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>2.9%</TD><TD class=relative id=r5c6 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) noWrap>Type A</TD><TD class=relative id=r5c7 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>7.8 </TD><TD class=relative id=r5c8 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c9 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>532.5 </TD><TD class=relative id=r5c10 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c11 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Click here</TD><TR id=r6c0><TD class=relative id=r6c1 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Estonia</TD><TD class=relative id=r6c2 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Low</TD><TD class=relative id=r6c3 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>None</TD><TD class=relative id=r6c4 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>1</TD><TD class=relative id=r6c5 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>0%</TD><TD class=relative id=r6c6 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) noWrap>None</TD><TD class=relative id=r6c7 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>0.5</TD><TD class=relative id=r6c8 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c9 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>266.5</TD><TD class=relative id=r6c10 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c11 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Germany</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>18</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>11.1%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>772.0 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r9c0><TD class=relative id=r9c1 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Greece</TD><TD class=relative id=r9c2 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Low</TD><TD class=relative id=r9c3 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>None</TD><TD class=relative id=r9c4 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)></TD><TD class=relative id=r9c5 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)></TD><TD class=relative id=r9c6 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) noWrap></TD><TD class=relative id=r9c7 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) align=right>0.0</TD><TD class=relative id=r9c8 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>(graphs)</TD><TD class=relative id=r9c9 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) noWrap></TD><TD class=relative id=r9c10 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)></TD><TD class=relative id=r9c11 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Click here</TD><TR id=r10c0><TD class=relative id=r10c1 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Hungary</TD><TD class=relative id=r10c2 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Low</TD><TD class=relative id=r10c3 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>None</TD><TD class=relative id=r10c4 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>2</TD><TD class=relative id=r10c5 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>0%</TD><TD class=relative id=r10c6 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) noWrap>None</TD><TD class=relative id=r10c7 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>72.6</TD><TD class=relative id=r10c8 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c9 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>0.0</TD><TD class=relative id=r10c10 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c11 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Click here</TD><TR id=r11c0><TD class=relative id=r11c1 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Ireland</TD><TD class=relative id=r11c2 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Low</TD><TD class=relative id=r11c3 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>None</TD><TD class=relative id=r11c4 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>1</TD><TD class=relative id=r11c5 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>0%</TD><TD class=relative id=r11c6 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) noWrap>None</TD><TD class=relative id=r11c7 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>5.6 </TD><TD class=relative id=r11c8 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c9 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>0.0 </TD><TD class=relative id=r11c10 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c11 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Click here</TD><TR id=r13c0><TD class=relative id=r13c1 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Latvia</TD><TD class=relative id=r13c2 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Low</TD><TD class=relative id=r13c3 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>None</TD><TD class=relative id=r13c4 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0</TD><TD class=relative id=r13c5 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0%</TD><TD class=relative id=r13c6 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) noWrap>None</TD><TD class=relative id=r13c7 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>0.0 </TD><TD class=relative id=r13c8 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c9 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>868.6 </TD><TD class=relative id=r13c10 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c11 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Click here</TD><TR id=r14c0><TD class=relative id=r14c1 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Lithuania</TD><TD class=relative id=r14c2 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Low</TD><TD class=relative id=r14c3 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>None</TD><TD class=relative id=r14c4 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0</TD><TD class=relative id=r14c5 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0%</TD><TD class=relative id=r14c6 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) noWrap>None</TD><TD class=relative id=r14c7 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>0.2 </TD><TD class=relative id=r14c8 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c9 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>476.8 </TD><TD class=relative id=r14c10 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c11 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Click here</TD><TR id=r15c0><TD class=relative id=r15c1 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>Luxembourg</TD><TD class=relative id=r15c2 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>Low</TD><TD class=relative id=r15c3 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>None</TD><TD class=relative id=r15c4 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>3</TD><TD class=relative id=r15c5 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>0%</TD><TD class=relative id=r15c6 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11) noWrap>None</TD><TD class=relative id=r15c7 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11) align=right>0.0 </TD><TD class=relative id=r15c8 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>(graphs)</TD><TD class=relative id=r15c9 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11) align=right>2418.4 </TD><TD class=relative id=r15c10 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>(graphs)</TD><TD class=relative id=r15c11 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>Click here</TD><TR id=r17c0><TD class=relative id=r17c1 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Netherlands</TD><TD class=relative id=r17c2 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Low</TD><TD class=relative id=r17c3 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>None</TD><TD class=relative id=r17c4 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>7</TD><TD class=relative id=r17c5 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>0%</TD><TD class=relative id=r17c6 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) noWrap>None</TD><TD class=relative id=r17c7 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>21.7 </TD><TD class=relative id=r17c8 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c9 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>0.0 </TD><TD class=relative id=r17c10 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c11 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Click here</TD><TR id=r18c0><TD class=relative id=r18c1 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Northern Ireland</TD><TD class=relative id=r18c2 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Low</TD><TD class=relative id=r18c3 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>None</TD><TD class=relative id=r18c4 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>4</TD><TD class=relative id=r18c5 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>0%</TD><TD class=relative id=r18c6 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) noWrap>None</TD><TD class=relative id=r18c7 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) align=right>22.4 </TD><TD class=relative id=r18c8 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>(graphs)</TD><TD class=relative id=r18c9 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) align=right>0.0 </TD><TD class=relative id=r18c10 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>(graphs)</TD><TD class=relative id=r18c11 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Click here</TD><TR id=r19c0><TD class=relative id=r19c1 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Norway</TD><TD class=relative id=r19c2 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Low</TD><TD class=relative id=r19c3 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>None</TD><TD class=relative id=r19c4 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>0</TD><TD class=relative id=r19c5 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>0%</TD><TD class=relative id=r19c6 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) noWrap>None</TD><TD class=relative id=r19c7 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>28.1 </TD><TD class=relative id=r19c8 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c9 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>0.0 </TD><TD class=relative id=r19c10 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c11 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Click here</TD><TR id=r20c0><TD class=relative id=r20c1 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Poland</TD><TD class=relative id=r20c2 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Low</TD><TD class=relative id=r20c3 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>None</TD><TD class=relative id=r20c4 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>15</TD><TD class=relative id=r20c5 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>0%</TD><TD class=relative id=r20c6 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) noWrap>None</TD><TD class=relative id=r20c7 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>41.0 </TD><TD class=relative id=r20c8 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c9 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>0.0 </TD><TD class=relative id=r20c10 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c11 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Click here</TD><TR id=r21c0><TD class=relative id=r21c1 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Portugal</TD><TD class=relative id=r21c2 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Low</TD><TD class=relative id=r21c3 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>None</TD><TD class=relative id=r21c4 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>1</TD><TD class=relative id=r21c5 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>0%</TD><TD class=relative id=r21c6 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) noWrap>None</TD><TD class=relative id=r21c7 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>0.0 </TD><TD class=relative id=r21c8 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c9 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>0.0 </TD><TD class=relative id=r21c10 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c11 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Click here</TD><TR id=r22c0><TD class=relative id=r22c1 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Romania</TD><TD class=relative id=r22c2 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Low</TD><TD class=relative id=r22c3 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>None</TD><TD class=relative id=r22c4 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>10</TD><TD class=relative id=r22c5 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>0%</TD><TD class=relative id=r22c6 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) noWrap>None</TD><TD class=relative id=r22c7 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>0.3 </TD><TD class=relative id=r22c8 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c9 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>978.2 </TD><TD class=relative id=r22c10 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c11 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Click here</TD><TR id=r24c0><TD class=relative id=r24c1 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Serbia</TD><TD class=relative id=r24c2 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Low</TD><TD class=relative id=r24c3 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>None</TD><TD class=relative id=r24c4 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0</TD><TD class=relative id=r24c5 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0%</TD><TD class=relative id=r24c6 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) noWrap>None</TD><TD class=relative id=r24c7 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>49.7</TD><TD class=relative id=r24c8 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c9 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>0.0</TD><TD class=relative id=r24c10 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c11 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Click here</TD><TR id=r25c0><TD class=relative id=r25c1 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Slovakia</TD><TD class=relative id=r25c2 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Low</TD><TD class=relative id=r25c3 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>None</TD><TD class=relative id=r25c4 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>1</TD><TD class=relative id=r25c5 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>0%</TD><TD class=relative id=r25c6 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) noWrap>None</TD><TD class=relative id=r25c7 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>181.2 </TD><TD class=relative id=r25c8 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c9 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>1561.9 </TD><TD class=relative id=r25c10 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c11 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Click here</TD><TR id=r26c0><TD class=relative id=r26c1 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Slovenia</TD><TD class=relative id=r26c2 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Low</TD><TD class=relative id=r26c3 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>None</TD><TD class=relative id=r26c4 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>4</TD><TD class=relative id=r26c5 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>0%</TD><TD class=relative id=r26c6 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) noWrap>None</TD><TD class=relative id=r26c7 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>0.0 </TD><TD class=relative id=r26c8 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c9 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>660.9 </TD><TD class=relative id=r26c10 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c11 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Click here</TD><TR id=r27c0><TD class=relative id=r27c1 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Spain</TD><TD class=relative id=r27c2 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Low</TD><TD class=relative id=r27c3 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>None</TD><TD class=relative id=r27c4 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>39</TD><TD class=relative id=r27c5 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>5.1%</TD><TD class=relative id=r27c6 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) noWrap>None</TD><TD class=relative id=r27c7 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>15.5 </TD><TD class=relative id=r27c8 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c9 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>0.0 </TD><TD class=relative id=r27c10 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c11 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Click here</TD><TR id=r28c0><TD class=relative id=r28c1 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Sweden</TD><TD class=relative id=r28c2 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Low</TD><TD class=relative id=r28c3 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>None</TD><TD class=relative id=r28c4 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>31</TD><TD class=relative id=r28c5 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>0%</TD><TD class=relative id=r28c6 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) noWrap>Type A</TD><TD class=relative id=r28c7 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>1.0 </TD><TD class=relative id=r28c8 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c9 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>0.0 </TD><TD class=relative id=r28c10 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c11 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Click here</TD><TR id=r30c0><TD class=relative id=r30c1 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>Wales</TD><TD class=relative id=r30c2 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>Low</TD><TD class=relative id=r30c3 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>None</TD><TD class=relative id=r30c4 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c5 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c6 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11) noWrap></TD><TD class=relative id=r30c7 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11) align=right>2.0 </TD><TD class=relative id=r30c8 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c9 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11) align=right>0.0 </TD><TD class=relative id=r30c10 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c11 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>Click here</TD><TR id=r31c0><TD class=relative id=r31c1 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>Europe</TD><TD class=relative id=r31c2 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c3 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c4 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>209</TD><TD class=relative id=r31c5 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>2.4%</TD><TD class=relative id=r31c6 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c7 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11) align=right></TD><TD class=relative id=r31c8 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c9 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c10 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c11 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>Click here</TD><TR><TD bgColor=#808080 colSpan=14>
</TD></TR><TR id=r2c0><TD class=relative id=r2c1 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Belgium</TD><TD class=relative id=r2c2 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Low</TD><TD class=relative id=r2c3 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>None</TD><TD class=relative id=r2c4 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>15</TD><TD class=relative id=r2c5 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>0%</TD><TD class=relative id=r2c6 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) noWrap>None</TD><TD class=relative id=r2c7 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>38.6</TD><TD class=relative id=r2c8 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c9 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11) align=right>270.3</TD><TD class=relative id=r2c10 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>(graphs)</TD><TD class=relative id=r2c11 onmouseover=crossRow_over(2,11) onmouseout=crossRow_out(2,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Bulgaria</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>0%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>589.0 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r3c0><TD class=relative id=r3c1 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Czech Republic</TD><TD class=relative id=r3c2 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Low</TD><TD class=relative id=r3c3 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>None</TD><TD class=relative id=r3c4 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>20</TD><TD class=relative id=r3c5 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>0%</TD><TD class=relative id=r3c6 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) noWrap>None</TD><TD class=relative id=r3c7 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>22.6 </TD><TD class=relative id=r3c8 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c9 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11) align=right>935.7 </TD><TD class=relative id=r3c10 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>(graphs)</TD><TD class=relative id=r3c11 onmouseover=crossRow_over(3,11) onmouseout=crossRow_out(3,11)>Click here</TD><TR id=r4c0><TD class=relative id=r4c1 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Denmark</TD><TD class=relative id=r4c2 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Low</TD><TD class=relative id=r4c3 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>None</TD><TD class=relative id=r4c4 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>2</TD><TD class=relative id=r4c5 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>0%</TD><TD class=relative id=r4c6 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) noWrap>None</TD><TD class=relative id=r4c7 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) align=right>9.9 </TD><TD class=relative id=r4c8 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>(graphs)</TD><TD class=relative id=r4c9 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11) align=right>0.0 </TD><TD class=relative id=r4c10 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>(graphs)</TD><TD class=relative id=r4c11 onmouseover=crossRow_over(4,11) onmouseout=crossRow_out(4,11)>Click here</TD><TR id=r5c0><TD class=relative id=r5c1 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>England</TD><TD class=relative id=r5c2 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Low</TD><TD class=relative id=r5c3 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Sporadic</TD><TD class=relative id=r5c4 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>35</TD><TD class=relative id=r5c5 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>2.9%</TD><TD class=relative id=r5c6 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) noWrap>Type A</TD><TD class=relative id=r5c7 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>7.8 </TD><TD class=relative id=r5c8 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c9 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11) align=right>532.5 </TD><TD class=relative id=r5c10 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>(graphs)</TD><TD class=relative id=r5c11 onmouseover=crossRow_over(5,11) onmouseout=crossRow_out(5,11)>Click here</TD><TR id=r6c0><TD class=relative id=r6c1 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Estonia</TD><TD class=relative id=r6c2 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Low</TD><TD class=relative id=r6c3 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>None</TD><TD class=relative id=r6c4 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>1</TD><TD class=relative id=r6c5 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>0%</TD><TD class=relative id=r6c6 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) noWrap>None</TD><TD class=relative id=r6c7 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>0.5</TD><TD class=relative id=r6c8 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c9 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11) align=right>266.5</TD><TD class=relative id=r6c10 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>(graphs)</TD><TD class=relative id=r6c11 onmouseover=crossRow_over(6,11) onmouseout=crossRow_out(6,11)>Click here</TD><TR id=r8c0><TD class=relative id=r8c1 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Germany</TD><TD class=relative id=r8c2 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Low</TD><TD class=relative id=r8c3 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>None</TD><TD class=relative id=r8c4 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>18</TD><TD class=relative id=r8c5 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>11.1%</TD><TD class=relative id=r8c6 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) noWrap>None</TD><TD class=relative id=r8c7 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>0.0 </TD><TD class=relative id=r8c8 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c9 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11) align=right>772.0 </TD><TD class=relative id=r8c10 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>(graphs)</TD><TD class=relative id=r8c11 onmouseover=crossRow_over(8,11) onmouseout=crossRow_out(8,11)>Click here</TD><TR id=r9c0><TD class=relative id=r9c1 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Greece</TD><TD class=relative id=r9c2 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Low</TD><TD class=relative id=r9c3 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>None</TD><TD class=relative id=r9c4 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)></TD><TD class=relative id=r9c5 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)></TD><TD class=relative id=r9c6 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) noWrap></TD><TD class=relative id=r9c7 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) align=right>0.0</TD><TD class=relative id=r9c8 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>(graphs)</TD><TD class=relative id=r9c9 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11) noWrap></TD><TD class=relative id=r9c10 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)></TD><TD class=relative id=r9c11 onmouseover=crossRow_over(9,11) onmouseout=crossRow_out(9,11)>Click here</TD><TR id=r10c0><TD class=relative id=r10c1 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Hungary</TD><TD class=relative id=r10c2 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Low</TD><TD class=relative id=r10c3 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>None</TD><TD class=relative id=r10c4 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>2</TD><TD class=relative id=r10c5 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>0%</TD><TD class=relative id=r10c6 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) noWrap>None</TD><TD class=relative id=r10c7 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>72.6</TD><TD class=relative id=r10c8 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c9 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11) align=right>0.0</TD><TD class=relative id=r10c10 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>(graphs)</TD><TD class=relative id=r10c11 onmouseover=crossRow_over(10,11) onmouseout=crossRow_out(10,11)>Click here</TD><TR id=r11c0><TD class=relative id=r11c1 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Ireland</TD><TD class=relative id=r11c2 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Low</TD><TD class=relative id=r11c3 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>None</TD><TD class=relative id=r11c4 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>1</TD><TD class=relative id=r11c5 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>0%</TD><TD class=relative id=r11c6 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) noWrap>None</TD><TD class=relative id=r11c7 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>5.6 </TD><TD class=relative id=r11c8 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c9 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11) align=right>0.0 </TD><TD class=relative id=r11c10 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>(graphs)</TD><TD class=relative id=r11c11 onmouseover=crossRow_over(11,11) onmouseout=crossRow_out(11,11)>Click here</TD><TR id=r13c0><TD class=relative id=r13c1 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Latvia</TD><TD class=relative id=r13c2 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Low</TD><TD class=relative id=r13c3 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>None</TD><TD class=relative id=r13c4 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0</TD><TD class=relative id=r13c5 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>0%</TD><TD class=relative id=r13c6 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) noWrap>None</TD><TD class=relative id=r13c7 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>0.0 </TD><TD class=relative id=r13c8 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c9 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11) align=right>868.6 </TD><TD class=relative id=r13c10 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>(graphs)</TD><TD class=relative id=r13c11 onmouseover=crossRow_over(13,11) onmouseout=crossRow_out(13,11)>Click here</TD><TR id=r14c0><TD class=relative id=r14c1 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Lithuania</TD><TD class=relative id=r14c2 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Low</TD><TD class=relative id=r14c3 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>None</TD><TD class=relative id=r14c4 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0</TD><TD class=relative id=r14c5 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>0%</TD><TD class=relative id=r14c6 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) noWrap>None</TD><TD class=relative id=r14c7 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>0.2 </TD><TD class=relative id=r14c8 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c9 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11) align=right>476.8 </TD><TD class=relative id=r14c10 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>(graphs)</TD><TD class=relative id=r14c11 onmouseover=crossRow_over(14,11) onmouseout=crossRow_out(14,11)>Click here</TD><TR id=r15c0><TD class=relative id=r15c1 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>Luxembourg</TD><TD class=relative id=r15c2 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>Low</TD><TD class=relative id=r15c3 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>None</TD><TD class=relative id=r15c4 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>3</TD><TD class=relative id=r15c5 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>0%</TD><TD class=relative id=r15c6 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11) noWrap>None</TD><TD class=relative id=r15c7 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11) align=right>0.0 </TD><TD class=relative id=r15c8 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>(graphs)</TD><TD class=relative id=r15c9 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11) align=right>2418.4 </TD><TD class=relative id=r15c10 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>(graphs)</TD><TD class=relative id=r15c11 onmouseover=crossRow_over(15,11) onmouseout=crossRow_out(15,11)>Click here</TD><TR id=r17c0><TD class=relative id=r17c1 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Netherlands</TD><TD class=relative id=r17c2 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Low</TD><TD class=relative id=r17c3 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>None</TD><TD class=relative id=r17c4 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>7</TD><TD class=relative id=r17c5 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>0%</TD><TD class=relative id=r17c6 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) noWrap>None</TD><TD class=relative id=r17c7 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>21.7 </TD><TD class=relative id=r17c8 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c9 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11) align=right>0.0 </TD><TD class=relative id=r17c10 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>(graphs)</TD><TD class=relative id=r17c11 onmouseover=crossRow_over(17,11) onmouseout=crossRow_out(17,11)>Click here</TD><TR id=r18c0><TD class=relative id=r18c1 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Northern Ireland</TD><TD class=relative id=r18c2 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Low</TD><TD class=relative id=r18c3 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>None</TD><TD class=relative id=r18c4 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>4</TD><TD class=relative id=r18c5 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>0%</TD><TD class=relative id=r18c6 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) noWrap>None</TD><TD class=relative id=r18c7 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) align=right>22.4 </TD><TD class=relative id=r18c8 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>(graphs)</TD><TD class=relative id=r18c9 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11) align=right>0.0 </TD><TD class=relative id=r18c10 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>(graphs)</TD><TD class=relative id=r18c11 onmouseover=crossRow_over(18,11) onmouseout=crossRow_out(18,11)>Click here</TD><TR id=r19c0><TD class=relative id=r19c1 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Norway</TD><TD class=relative id=r19c2 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Low</TD><TD class=relative id=r19c3 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>None</TD><TD class=relative id=r19c4 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>0</TD><TD class=relative id=r19c5 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>0%</TD><TD class=relative id=r19c6 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) noWrap>None</TD><TD class=relative id=r19c7 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>28.1 </TD><TD class=relative id=r19c8 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c9 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11) align=right>0.0 </TD><TD class=relative id=r19c10 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>(graphs)</TD><TD class=relative id=r19c11 onmouseover=crossRow_over(19,11) onmouseout=crossRow_out(19,11)>Click here</TD><TR id=r20c0><TD class=relative id=r20c1 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Poland</TD><TD class=relative id=r20c2 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Low</TD><TD class=relative id=r20c3 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>None</TD><TD class=relative id=r20c4 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>15</TD><TD class=relative id=r20c5 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>0%</TD><TD class=relative id=r20c6 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) noWrap>None</TD><TD class=relative id=r20c7 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>41.0 </TD><TD class=relative id=r20c8 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c9 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11) align=right>0.0 </TD><TD class=relative id=r20c10 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>(graphs)</TD><TD class=relative id=r20c11 onmouseover=crossRow_over(20,11) onmouseout=crossRow_out(20,11)>Click here</TD><TR id=r21c0><TD class=relative id=r21c1 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Portugal</TD><TD class=relative id=r21c2 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Low</TD><TD class=relative id=r21c3 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>None</TD><TD class=relative id=r21c4 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>1</TD><TD class=relative id=r21c5 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>0%</TD><TD class=relative id=r21c6 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) noWrap>None</TD><TD class=relative id=r21c7 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>0.0 </TD><TD class=relative id=r21c8 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c9 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11) align=right>0.0 </TD><TD class=relative id=r21c10 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>(graphs)</TD><TD class=relative id=r21c11 onmouseover=crossRow_over(21,11) onmouseout=crossRow_out(21,11)>Click here</TD><TR id=r22c0><TD class=relative id=r22c1 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Romania</TD><TD class=relative id=r22c2 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Low</TD><TD class=relative id=r22c3 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>None</TD><TD class=relative id=r22c4 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>10</TD><TD class=relative id=r22c5 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>0%</TD><TD class=relative id=r22c6 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) noWrap>None</TD><TD class=relative id=r22c7 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>0.3 </TD><TD class=relative id=r22c8 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c9 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11) align=right>978.2 </TD><TD class=relative id=r22c10 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>(graphs)</TD><TD class=relative id=r22c11 onmouseover=crossRow_over(22,11) onmouseout=crossRow_out(22,11)>Click here</TD><TR id=r24c0><TD class=relative id=r24c1 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Serbia</TD><TD class=relative id=r24c2 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Low</TD><TD class=relative id=r24c3 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>None</TD><TD class=relative id=r24c4 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0</TD><TD class=relative id=r24c5 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>0%</TD><TD class=relative id=r24c6 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) noWrap>None</TD><TD class=relative id=r24c7 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>49.7</TD><TD class=relative id=r24c8 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c9 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11) align=right>0.0</TD><TD class=relative id=r24c10 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>(graphs)</TD><TD class=relative id=r24c11 onmouseover=crossRow_over(24,11) onmouseout=crossRow_out(24,11)>Click here</TD><TR id=r25c0><TD class=relative id=r25c1 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Slovakia</TD><TD class=relative id=r25c2 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Low</TD><TD class=relative id=r25c3 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>None</TD><TD class=relative id=r25c4 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>1</TD><TD class=relative id=r25c5 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>0%</TD><TD class=relative id=r25c6 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) noWrap>None</TD><TD class=relative id=r25c7 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>181.2 </TD><TD class=relative id=r25c8 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c9 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11) align=right>1561.9 </TD><TD class=relative id=r25c10 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>(graphs)</TD><TD class=relative id=r25c11 onmouseover=crossRow_over(25,11) onmouseout=crossRow_out(25,11)>Click here</TD><TR id=r26c0><TD class=relative id=r26c1 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Slovenia</TD><TD class=relative id=r26c2 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Low</TD><TD class=relative id=r26c3 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>None</TD><TD class=relative id=r26c4 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>4</TD><TD class=relative id=r26c5 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>0%</TD><TD class=relative id=r26c6 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) noWrap>None</TD><TD class=relative id=r26c7 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>0.0 </TD><TD class=relative id=r26c8 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c9 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11) align=right>660.9 </TD><TD class=relative id=r26c10 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>(graphs)</TD><TD class=relative id=r26c11 onmouseover=crossRow_over(26,11) onmouseout=crossRow_out(26,11)>Click here</TD><TR id=r27c0><TD class=relative id=r27c1 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Spain</TD><TD class=relative id=r27c2 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Low</TD><TD class=relative id=r27c3 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>None</TD><TD class=relative id=r27c4 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>39</TD><TD class=relative id=r27c5 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>5.1%</TD><TD class=relative id=r27c6 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) noWrap>None</TD><TD class=relative id=r27c7 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>15.5 </TD><TD class=relative id=r27c8 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c9 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11) align=right>0.0 </TD><TD class=relative id=r27c10 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>(graphs)</TD><TD class=relative id=r27c11 onmouseover=crossRow_over(27,11) onmouseout=crossRow_out(27,11)>Click here</TD><TR id=r28c0><TD class=relative id=r28c1 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Sweden</TD><TD class=relative id=r28c2 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Low</TD><TD class=relative id=r28c3 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>None</TD><TD class=relative id=r28c4 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>31</TD><TD class=relative id=r28c5 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>0%</TD><TD class=relative id=r28c6 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) noWrap>Type A</TD><TD class=relative id=r28c7 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>1.0 </TD><TD class=relative id=r28c8 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c9 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11) align=right>0.0 </TD><TD class=relative id=r28c10 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>(graphs)</TD><TD class=relative id=r28c11 onmouseover=crossRow_over(28,11) onmouseout=crossRow_out(28,11)>Click here</TD><TR id=r30c0><TD class=relative id=r30c1 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>Wales</TD><TD class=relative id=r30c2 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>Low</TD><TD class=relative id=r30c3 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>None</TD><TD class=relative id=r30c4 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c5 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)></TD><TD class=relative id=r30c6 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11) noWrap></TD><TD class=relative id=r30c7 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11) align=right>2.0 </TD><TD class=relative id=r30c8 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c9 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11) align=right>0.0 </TD><TD class=relative id=r30c10 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>(graphs)</TD><TD class=relative id=r30c11 onmouseover=crossRow_over(30,11) onmouseout=crossRow_out(30,11)>Click here</TD><TR id=r31c0><TD class=relative id=r31c1 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>Europe</TD><TD class=relative id=r31c2 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c3 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c4 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>209</TD><TD class=relative id=r31c5 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>2.4%</TD><TD class=relative id=r31c6 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c7 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11) align=right></TD><TD class=relative id=r31c8 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c9 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11) noWrap></TD><TD class=relative id=r31c10 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)></TD><TD class=relative id=r31c11 onmouseover=crossRow_over(31,11) onmouseout=crossRow_out(31,11)>Click here</TD><TR><TD bgColor=#808080 colSpan=14> </TD></TR><TR><TD colSpan=14>Preliminary data</TD><TR><TD colSpan=14>
</TD></TR><TR><TD colSpan=14>Preliminary data</TD><TR><TD colSpan=14> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1>
</TD></TR></TBODY></TABLE><TABLE cellSpacing=0 cellPadding=0 width=782 border=0><TBODY><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=#1d92ff height=5>
</TD><TD width=780 bgColor=#1d92ff height=5> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD><TABLE><TBODY><TR><TD>The bulletin text was written by an editorial team at the European Centre for Disease Prevention and Control (ECDC) and the Community Network of Reference Laboratories for Human Influenza in Europe (CNRL). Team members are Flaviu Plata, Phillip Zucs and Bruno Ciancio from ECDC, and Adam, Meijer Rod Daniels Alan Hay and Maria Zambon from CNRL. The bulletin text was reviewed by Olav Hungnes (Norwegian Institute of Public Health, Oslo, Norway), and Anne Mazick (Statens Serum Institut, Copenhagen, Denmark) on behalf of the EISS members.
</TD><TD width=780 bgColor=white><TABLE><TBODY><TR><TD><TABLE><TBODY><TR><TD>The bulletin text was written by an editorial team at the European Centre for Disease Prevention and Control (ECDC) and the Community Network of Reference Laboratories for Human Influenza in Europe (CNRL). Team members are Flaviu Plata, Phillip Zucs and Bruno Ciancio from ECDC, and Adam, Meijer Rod Daniels Alan Hay and Maria Zambon from CNRL. The bulletin text was reviewed by Olav Hungnes (Norwegian Institute of Public Health, Oslo, Norway), and Anne Mazick (Statens Serum Institut, Copenhagen, Denmark) on behalf of the EISS members. </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD width=780 bgColor=black height=1>
</TD><TD width=780 bgColor=black height=1> </TD><TD width=1 bgColor=black height=1>
</TD><TD width=1 bgColor=black height=1> </TD></TR><TR><TD width=1 bgColor=black height=1>
</TD></TR><TR><TD width=1 bgColor=black height=1> </TD><TD align=middle width=780 bgColor=#0063c8>EISS : Weekly Electronic Bulletin</TD></TR></TBODY></TABLE>
</TD><TD align=middle width=780 bgColor=#0063c8>EISS : Weekly Electronic Bulletin</TD></TR></TBODY></TABLE>
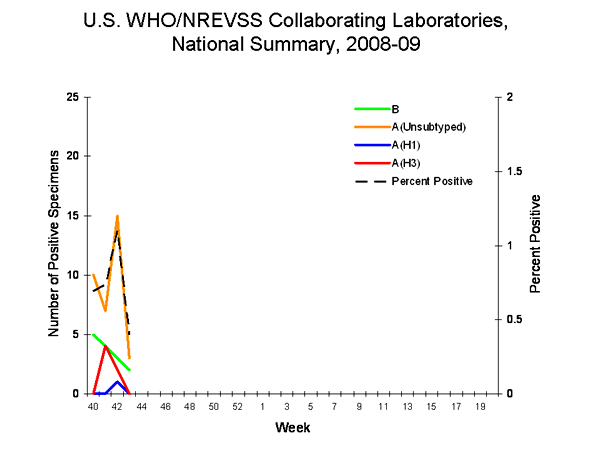
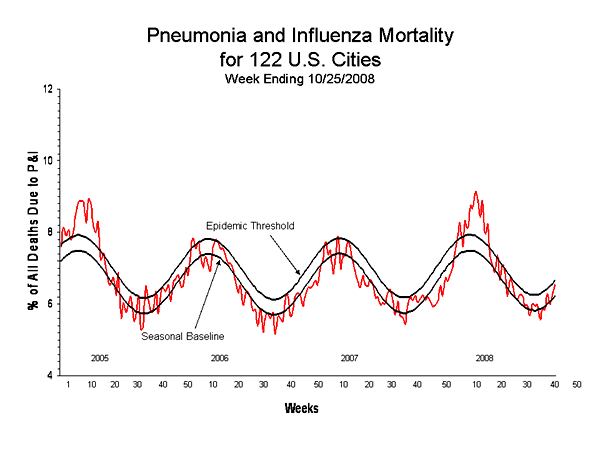
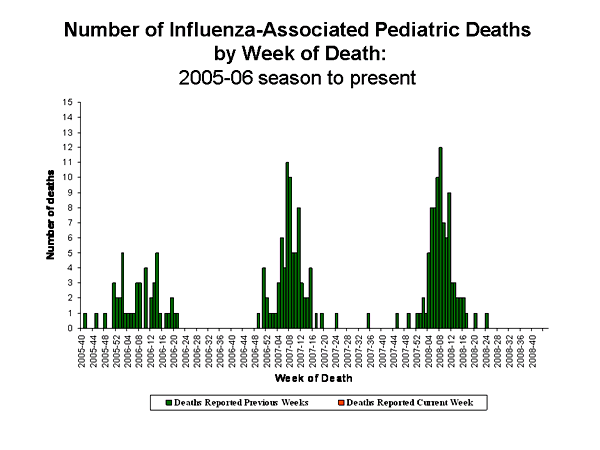
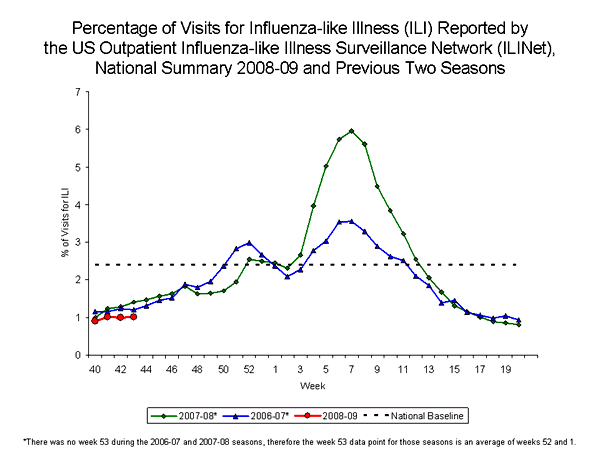
Comment