Interim Guidance for Specimen Collection, Transport, Testing, and Submission for Persons Under Investigation for Ebola Virus Disease in the United States
Updated: August 26, 2014
Who this is for: Laboratorians and other healthcare personnel handling specimens for Ebola testing
What: CDC provides updated guidance for collecting specimens correctly, transporting and testing specimens from persons under investigation for Ebola virus disease.
How to use: This guidance should be used to explain exactly the biosafety requirements for how to collect and perform routine testing of specimens to staff working in laboratory and healthcare settings.
Key Points
Background
CDC is working with the World Health Organization (WHO), the ministries of health and other international organizations in response to an outbreak of Ebola virus disease (EVD) in West Africa, which was first reported in late March 2014. For the latest information on the outbreak, please see the 2014 Ebola Outbreak in West Africa highlights. This is the largest outbreak of EVD ever documented and the first recorded in West Africa.
EVD is one of numerous viral hemorrhagic fevers (VHF). It is a severe, often fatal disease in human and nonhuman primates. Ebola virus is spread by direct contact with the blood or body fluids (such as urine, saliva, feces, vomit and semen) of an infected person or by being exposed to objects that have been contaminated with infected blood or body fluids. The incubation period is usually 8?10 days (rarely ranging from 2 to 21 days). Patients can transmit the virus once symptoms appear and through the later stages of disease, as well as postmortem.
U.S. hospitals can safely manage a patient with EVD by using all recommended isolation and infection control procedures. Standard, contact, and droplet precautions are recommended for management of hospitalized patients with known or suspected EVD. Similarly, U.S. clinical laboratories can safely handle specimens from these patients by strict adherence to precautions and practices specifically designed for bloodborne pathogens in the laboratory environment. However, Ebola has an apparent low infectious dose, the potential of high virus titers in the blood of ill patients, and can result in severe disease. Therefore, it is essential that laboratorians, supervisors, and other workers review laboratory safety procedures and guidelines to make sure to follow these biosafety recommendations. Following these guidelines U.S. hospitals and clinical laboratories have safely managed a number of VHF patients including cases of Lassa fever and Marburg virus (a closely related virus to Ebola).<SUP>1-4</SUP>
Potentially infectious diagnostic specimens are routinely handled and tested in U.S. laboratories in a safe manner, by closely following the standard safety precautions below.
<!-- -->Top of Page

Printable factsheet: Interim Guidance for Specimen Collection, Transport, Testing, and Submission for Patients with Suspected Infection with Ebola Virus Disease[PDF - 1 page]
Infection Control When Collecting and Handling Specimens
All laboratorians and other healthcare personnel collecting or handling specimens must follow established standards compliant with the OSHA bloodborne pathogens standard, which includes blood and other potentially infectious materials. These standards include wearing appropriate personal protective equipment (PPE) and following all safety rules for all specimens regardless of whether they are identified as being infectious.
Recommendations for risk assessment to staff: Risk assessments should be conducted by each laboratory director, biosafety officer, or other responsible personnel to determine the potential for sprays, splashes, or aerosols generated from laboratory procedures. They should adjust, as needed, PPE requirements, practices, and safety equipment controls to protect the laboratorian?s skin, eyes, and mucous membranes.
Recommendations for specimen collection by staff: Any person collecting specimens from a patient with a case of suspected Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth Additional PPE may be required in certain situations.
Recommendations for laboratory testing by staff: Any person testing specimens from a patient with a suspected case of Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth, and as an added precaution use a certified class II Biosafety cabinet or Plexiglass splash guard with PPE to protect skin and mucous membranes. All manufacturer-installed safety features for laboratory instruments should be used.
<!-- -->Top of Page
Specimen Handling for Routine Laboratory Testing (not for Ebola Diagnosis)
Routine laboratory testing includes traditional chemistry, hematology, and other laboratory testing used to support and treat patients. Precautions as described above offer appropriate protection for healthcare personnel performing laboratory testing on specimens from patients with suspected infection with Ebola virus. These precautions include both manufacturer installed safety features for instruments and the laboratory environment as well as PPE specified in the box above.
Environmental Cleaning and Disinfection
See the Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus for recommendations regarding the cleaning and disinfection of patient care area surfaces including the management of blood and body fluid spills. These recommendations also apply to cleaning and disinfecting in a laboratory where specimens are being processed from persons under investigation, or with probable or confirmed Ebola virus infections.
In the case of a spill in the laboratory, the basic principles for blood or body substance spill management are outlined in the United States OSHA Blood Borne Pathogens Standards. There are no disinfection products with specific label claims against the Ebola virus. Enveloped viruses such as Ebola are susceptible to a broad range of hospital disinfectants used to disinfect hard, non-porous surfaces. In contrast, non-enveloped viruses are more resistant to disinfectants. As an added precaution, use a disinfectant with a higher potency than what is normally required for an enveloped virus to disinfect potentially Ebola-contaminated surfaces, such as. EPA-registered hospital disinfectants with label claims against non-enveloped viruses (e.g., norovirus, rotavirus, adenovirus, poliovirus) are broadly antiviral and capable of inactivating both enveloped and non-enveloped viruses.
Management of Laboratory Waste
Waste generated during laboratory testing should be placed in leak-proof containment and discarded as regulated medical waste. To minimize contamination of the exterior of the waste bag, place this bag in a rigid waste container designed for this use. If available, steam sterilization (autoclave) or incineration as a waste treatment process can inactivate the virus and reduces waste volume. For equipment that drains directly into the sewer system, the United States sanitary sewer system handling processes (e.g., anaerobic digestion, composting, disinfection) are designed to safely inactivate infectious agents. However, check with your state's regulated medical waste program for more guidance and coordinate your waste management activities for the laboratory area with your medical waste contractor. <SUP>5</SUP>
CDC Division of Select Agents and Toxins (DSAT) Considerations
If these guidelines for the collection, transport, and testing of specimens from suspected or confirmed Ebola patients are followed, waste generated during the handling and testing of such specimens and which is properly disposed would not be subject to Federal select agent regulations (See the exclusion provision 42 CFR ? 73.3(d)(1)). However, this exclusion would not apply to any facility or laboratory that intentionally collected or otherwise extracted the Ebola virus from waste generated during the delivery of patient care.
<!-- -->Top of Page
Transporting Specimens within the Hospital / Institution
In compliance with 29 CFR 1910.1030, specimens should be placed in a durable, leak-proof secondary container for transport within a facility. To reduce the risk of breakage or leaks, do not use any pneumatic tube system for transporting suspected EVD specimens.
When Specimens Should Be Collected for Ebola Testing at CDC
Ebola virus is detected in blood only after the onset of symptoms, usually fever. It may take up to 3 days after symptoms appear for the virus to reach detectable levels. Virus is generally detectable by real-time RT-PCR from 3-10 days after symptoms appear.
Specimens ideally should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an Ebola exposure. However, if the onset of symptoms is <3 days, a later specimen may be needed to completely rule-out Ebola virus, if the first specimen tests negative.
Preferred Specimens for Ebola Testing at CDC
A minimum volume of 4mL whole blood in plastic collection tubes can be used to submit specimens for testing for Ebola virus. Do not submit specimens to CDC in glass containers or in heparinized tubes. Whole blood preserved with EDTA is preferred but whole blood preserved with; sodium polyanethol sulfonate (SPS), citrate, or with clot activator is acceptable. It is not necessary to separate and remove serum or plasma from the primary collection container. Specimens should be immediately stored or transported at 2-8?C or frozen on cold-packs to the CDC. Specimens other than blood may be submitted upon consult with the CDC by calling the Emergency Operations Center at 770-488-7100.
Standard labeling should be applied for each specimen. The requested test only needs to be identified on the requisition and CDC specimen submission forms.
Storing Clinical Specimens for Ebola Testing at CDC
Short-term storage of specimens prior to shipping to CDC should be at 4?C or frozen.
Diagnostic Testing for Ebola Performed at CDC
Several diagnostic tests are available for detection of EVD. Acute infections will be confirmed using a real-time RT-PCR assay (CDC test directory code CDC -10309 Ebola Identification) in a CLIA-accredited laboratory. Virus isolation may also be attempted. Serologic testing for IgM and IgG antibodies will be completed for certain specimens and to monitor the immune response in confirmed EVD patients (#CDC-10310 Ebola Serology).
Lassa fever is also endemic in certain areas of West Africa and may show symptoms similar to early EVD. Diagnostic tests available at CDC include but are not limited to RT-PCR, antigen detection, and IgM serology all of which may be utilized to rule out Lassa fever in EVD-negative patients.
Packaging and Shipping Clinical Specimens to CDC
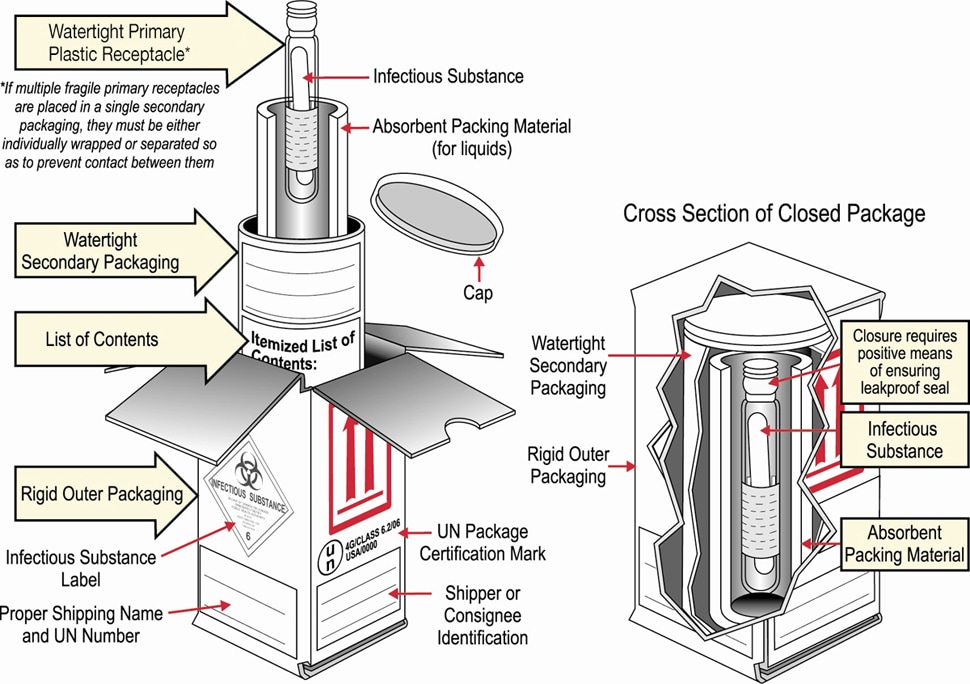
Packaging Diagram
Specimens collected for EVD testing should be packaged and shipped without attempting to open collection tubes or aliquot specimens.
The following steps should be used in submitting samples to CDC.
ATTN STAT LAB: VSPB, UNIT #70
1600 Clifton Road NE
Atlanta, GA 30333
Phone 770-488-7100
<!-- -->Top of Page
Occupational Health
Potential exposures to blood, body fluids and other infectious materials must be reported immediately according to your institution?s policies and procedures.
When to Contact CDC
CDC highly recommends contacting your state and/or local health department before contacting CDC.
CDC is available for consultation 24/7 at 770-488-7100.
CDC will continue to evaluate new information as it becomes available and will update this guidance as needed.
References:
Updated: August 26, 2014
Who this is for: Laboratorians and other healthcare personnel handling specimens for Ebola testing
What: CDC provides updated guidance for collecting specimens correctly, transporting and testing specimens from persons under investigation for Ebola virus disease.
How to use: This guidance should be used to explain exactly the biosafety requirements for how to collect and perform routine testing of specimens to staff working in laboratory and healthcare settings.
Key Points
- U.S. clinical laboratories can safely handle specimens from these potential Ebola patients by taking all required precautions and practices in the laboratory, specifically designed for pathogens spread in the blood.
- Risk assessments should be conducted by each laboratory director, biosafety officer, or other responsible person to determine the potential for sprays, splashes, or aerosol generated during laboratory procedures.
- Any person collecting specimens from a patient with suspected Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth.
- Anyone collecting specimens from a patient should follow the procedures below for transporting them through the healthcare facility, clean-up of spills, storing, packaging and shipping to CDC for testing.
Background
CDC is working with the World Health Organization (WHO), the ministries of health and other international organizations in response to an outbreak of Ebola virus disease (EVD) in West Africa, which was first reported in late March 2014. For the latest information on the outbreak, please see the 2014 Ebola Outbreak in West Africa highlights. This is the largest outbreak of EVD ever documented and the first recorded in West Africa.
EVD is one of numerous viral hemorrhagic fevers (VHF). It is a severe, often fatal disease in human and nonhuman primates. Ebola virus is spread by direct contact with the blood or body fluids (such as urine, saliva, feces, vomit and semen) of an infected person or by being exposed to objects that have been contaminated with infected blood or body fluids. The incubation period is usually 8?10 days (rarely ranging from 2 to 21 days). Patients can transmit the virus once symptoms appear and through the later stages of disease, as well as postmortem.
U.S. hospitals can safely manage a patient with EVD by using all recommended isolation and infection control procedures. Standard, contact, and droplet precautions are recommended for management of hospitalized patients with known or suspected EVD. Similarly, U.S. clinical laboratories can safely handle specimens from these patients by strict adherence to precautions and practices specifically designed for bloodborne pathogens in the laboratory environment. However, Ebola has an apparent low infectious dose, the potential of high virus titers in the blood of ill patients, and can result in severe disease. Therefore, it is essential that laboratorians, supervisors, and other workers review laboratory safety procedures and guidelines to make sure to follow these biosafety recommendations. Following these guidelines U.S. hospitals and clinical laboratories have safely managed a number of VHF patients including cases of Lassa fever and Marburg virus (a closely related virus to Ebola).<SUP>1-4</SUP>
Potentially infectious diagnostic specimens are routinely handled and tested in U.S. laboratories in a safe manner, by closely following the standard safety precautions below.
<!-- -->Top of Page

Printable factsheet: Interim Guidance for Specimen Collection, Transport, Testing, and Submission for Patients with Suspected Infection with Ebola Virus Disease[PDF - 1 page]
Infection Control When Collecting and Handling Specimens
All laboratorians and other healthcare personnel collecting or handling specimens must follow established standards compliant with the OSHA bloodborne pathogens standard, which includes blood and other potentially infectious materials. These standards include wearing appropriate personal protective equipment (PPE) and following all safety rules for all specimens regardless of whether they are identified as being infectious.
Recommendations for risk assessment to staff: Risk assessments should be conducted by each laboratory director, biosafety officer, or other responsible personnel to determine the potential for sprays, splashes, or aerosols generated from laboratory procedures. They should adjust, as needed, PPE requirements, practices, and safety equipment controls to protect the laboratorian?s skin, eyes, and mucous membranes.
Recommendations for specimen collection by staff: Any person collecting specimens from a patient with a case of suspected Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth Additional PPE may be required in certain situations.
Recommendations for laboratory testing by staff: Any person testing specimens from a patient with a suspected case of Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth, and as an added precaution use a certified class II Biosafety cabinet or Plexiglass splash guard with PPE to protect skin and mucous membranes. All manufacturer-installed safety features for laboratory instruments should be used.
<!-- -->Top of Page
Specimen Handling for Routine Laboratory Testing (not for Ebola Diagnosis)
Routine laboratory testing includes traditional chemistry, hematology, and other laboratory testing used to support and treat patients. Precautions as described above offer appropriate protection for healthcare personnel performing laboratory testing on specimens from patients with suspected infection with Ebola virus. These precautions include both manufacturer installed safety features for instruments and the laboratory environment as well as PPE specified in the box above.
Environmental Cleaning and Disinfection
See the Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus for recommendations regarding the cleaning and disinfection of patient care area surfaces including the management of blood and body fluid spills. These recommendations also apply to cleaning and disinfecting in a laboratory where specimens are being processed from persons under investigation, or with probable or confirmed Ebola virus infections.
In the case of a spill in the laboratory, the basic principles for blood or body substance spill management are outlined in the United States OSHA Blood Borne Pathogens Standards. There are no disinfection products with specific label claims against the Ebola virus. Enveloped viruses such as Ebola are susceptible to a broad range of hospital disinfectants used to disinfect hard, non-porous surfaces. In contrast, non-enveloped viruses are more resistant to disinfectants. As an added precaution, use a disinfectant with a higher potency than what is normally required for an enveloped virus to disinfect potentially Ebola-contaminated surfaces, such as. EPA-registered hospital disinfectants with label claims against non-enveloped viruses (e.g., norovirus, rotavirus, adenovirus, poliovirus) are broadly antiviral and capable of inactivating both enveloped and non-enveloped viruses.
Management of Laboratory Waste
Waste generated during laboratory testing should be placed in leak-proof containment and discarded as regulated medical waste. To minimize contamination of the exterior of the waste bag, place this bag in a rigid waste container designed for this use. If available, steam sterilization (autoclave) or incineration as a waste treatment process can inactivate the virus and reduces waste volume. For equipment that drains directly into the sewer system, the United States sanitary sewer system handling processes (e.g., anaerobic digestion, composting, disinfection) are designed to safely inactivate infectious agents. However, check with your state's regulated medical waste program for more guidance and coordinate your waste management activities for the laboratory area with your medical waste contractor. <SUP>5</SUP>
CDC Division of Select Agents and Toxins (DSAT) Considerations
If these guidelines for the collection, transport, and testing of specimens from suspected or confirmed Ebola patients are followed, waste generated during the handling and testing of such specimens and which is properly disposed would not be subject to Federal select agent regulations (See the exclusion provision 42 CFR ? 73.3(d)(1)). However, this exclusion would not apply to any facility or laboratory that intentionally collected or otherwise extracted the Ebola virus from waste generated during the delivery of patient care.
<!-- -->Top of Page
Transporting Specimens within the Hospital / Institution
In compliance with 29 CFR 1910.1030, specimens should be placed in a durable, leak-proof secondary container for transport within a facility. To reduce the risk of breakage or leaks, do not use any pneumatic tube system for transporting suspected EVD specimens.
When Specimens Should Be Collected for Ebola Testing at CDC
Ebola virus is detected in blood only after the onset of symptoms, usually fever. It may take up to 3 days after symptoms appear for the virus to reach detectable levels. Virus is generally detectable by real-time RT-PCR from 3-10 days after symptoms appear.
Specimens ideally should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an Ebola exposure. However, if the onset of symptoms is <3 days, a later specimen may be needed to completely rule-out Ebola virus, if the first specimen tests negative.
Preferred Specimens for Ebola Testing at CDC
A minimum volume of 4mL whole blood in plastic collection tubes can be used to submit specimens for testing for Ebola virus. Do not submit specimens to CDC in glass containers or in heparinized tubes. Whole blood preserved with EDTA is preferred but whole blood preserved with; sodium polyanethol sulfonate (SPS), citrate, or with clot activator is acceptable. It is not necessary to separate and remove serum or plasma from the primary collection container. Specimens should be immediately stored or transported at 2-8?C or frozen on cold-packs to the CDC. Specimens other than blood may be submitted upon consult with the CDC by calling the Emergency Operations Center at 770-488-7100.
Standard labeling should be applied for each specimen. The requested test only needs to be identified on the requisition and CDC specimen submission forms.
Storing Clinical Specimens for Ebola Testing at CDC
Short-term storage of specimens prior to shipping to CDC should be at 4?C or frozen.
Diagnostic Testing for Ebola Performed at CDC
Several diagnostic tests are available for detection of EVD. Acute infections will be confirmed using a real-time RT-PCR assay (CDC test directory code CDC -10309 Ebola Identification) in a CLIA-accredited laboratory. Virus isolation may also be attempted. Serologic testing for IgM and IgG antibodies will be completed for certain specimens and to monitor the immune response in confirmed EVD patients (#CDC-10310 Ebola Serology).
Lassa fever is also endemic in certain areas of West Africa and may show symptoms similar to early EVD. Diagnostic tests available at CDC include but are not limited to RT-PCR, antigen detection, and IgM serology all of which may be utilized to rule out Lassa fever in EVD-negative patients.
Packaging and Shipping Clinical Specimens to CDC

Packaging Diagram
Specimens collected for EVD testing should be packaged and shipped without attempting to open collection tubes or aliquot specimens.
The following steps should be used in submitting samples to CDC.
- Hospitals should follow their state and/or local health department procedures for notification and consultation for Ebola testing requests and prior to contacting CDC.
- NO specimens will be accepted without prior consultation. For consultation call the EOC at 770-488-7100.
- Contact your state and/or local health department and CDC to determine the proper category for shipment based on clinical history and risk assessment by CDC. State guidelines may differ and state or local health departments should be consulted prior to shipping.
- Email tracking number to EOCEVENT246@CDC.GOV.
- Do not ship for weekend delivery unless instructed by CDC.
- Ship to:
ATTN STAT LAB: VSPB, UNIT #70
1600 Clifton Road NE
Atlanta, GA 30333
Phone 770-488-7100
- Include the following information: your name, the patient's name, test(s) requested, date of collection, laboratory or accession number, and the type of specimen being shipped.
- Include the CDC Infectious Disease (CDC Form 50.34) and Viral Special Pathogens Branch specimen submission forms.
- On the outside of the box, specify how the specimen should be stored: refrigerated.
<!-- -->Top of Page
Occupational Health
Potential exposures to blood, body fluids and other infectious materials must be reported immediately according to your institution?s policies and procedures.
When to Contact CDC
CDC highly recommends contacting your state and/or local health department before contacting CDC.
CDC is available for consultation 24/7 at 770-488-7100.
CDC will continue to evaluate new information as it becomes available and will update this guidance as needed.
References:
- Amorosa V, et al.,. Imported Lassa fever, Pennsylvania, USA, 2010. Emerg Infect Dis. 2010 Oct;16(10):1598-600.
- Imported case of Marburg hemorrhagic fever - Colorado, 2008. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2009 Dec 18;58(49):1377-81.
- Timen A, et. al., Response to imported case of Marburg hemorrhagic fever, in the Netherlands. Emerg Infect Dis. 2009 Aug;15(8):1171-5.
- Centers for Disease Control and Prevention (CDC). Imported Lassa fever?New Jersey, 2004. MMWR Morb Mortal Wkly Rep. 2004 Oct 1;53(38):894-7.
- EPA Where You Live ? State Medical Waste Programs and Regulations (see: http://www.epa.gov/epawaste/nonhaz/industrial/medical/programs.htm ).
- http://www.cdc.gov/ncezid/dhcpp/vspb/pdf/specimen-submission.pdf[PDF - 2 pages]
- http://www.cdc.gov/ncezid/dhcpp/vspb/specimens.html
- http://www.cdc.gov/vhf/ebola/hcp/infection-prevention-and-control-recommendations.html
- http://content.govdelivery.com/accounts/USCDC/bulletins/c7bea0
- http://www.cdc.gov/hicpac/disinfection_sterilization/6_0disinfection.html
- http://www.cdc.gov/mmwr/pdf/other/su6101.pdf[PDF - 105 pages]
- http://www.cdc.gov/laboratory/specimen-submission/form.html