JJackson Seems I may be correct in some of my thinking, I hope not all. https://www.nature.com/articles/s414...0424-9#ref-CR9
Announcement
Collapse
No announcement yet.
Nature - SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion
Collapse
X
-
SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion- Xinling Wang,
- Wei Xu,
- Gaowei Hu,
- Shuai Xia,
- Zhiping Sun,
- Zezhong Liu,
- Youhua Xie,
- Rong Zhang,
- Shibo Jiang &
- Lu Lu
Cellular & Molecular Immunology (2020)Cite this article- 45k Accesses
- 2353 Altmetric
- Metricsdetails
COVID-19, the novel coronavirus disease caused by SARS-CoV-2 and outbroken at the end of 2019 in Wuhan, China,1 becomes a worldwide pandemic. SARS-CoV-2 belongs to the betacoronavirus genus and has 79.5% identity to SARS-CoV. SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as its host entry receptor.2 The clinical manifestations of COVID-19 include pneumonia, diarrhea, dyspnea, and multiple organ failure. Interestingly, lymphocytopenia, as a diagnostic indicator, is common in COVID-19 patients. Xiong et al. found upregulation of apoptosis, autophagy, and p53 pathways in PBMC of COVID-19 patients.3 Some studies reported that lymphocytopenia might be related to mortality, especially in patients with low levels of CD3+, CD4+, and CD8+ T lymphocytes.4,5 Lymphocytopenia was also found in the Middle East respiratory syndrome (MERS) cases. MERS-CoV can directly infect human primary T lymphocytes and induce T-cell apoptosis through extrinsic and intrinsic apoptosis pathways, but it cannot replicate in T lymphocytes.6 However, it is unclear whether SARS-CoV-2 can also infect T cells, resulting in lymphocytopenia.
To address this question, we evaluated the susceptibility of T lymphocytes to SARS-CoV-2 infection. To accomplish this, pseudotyped SARS-CoV and SARS-CoV-2 were packaged based on methods described previously.7 The pseudoviruses could infect permissive cells (293T/ACE2 and Huh7 cells) expressing the ACE2 receptor, but could not infect nonpermissive cells (HeLa cells) (Fig. 1a). We used pseudovirus with equal infectivity to 293T/ACE2 cells (Fig. 1c) to infect two T lymphocyte cell lines, MT-2 and A3.01, with very low, or close to negative, expression level of hACE2 mRNA (Fig. 1b). Surprisingly, over several replicates, we saw that the T-cell lines were significantly more sensitive to SARS-CoV-2 infection when compared with SARS-CoV (Fig. 1c). In other words, these results tell us that T lymphocytes may be more permissive to SARS-CoV-2 infection and less permissive for SARS-CoV infection, similar to the findings in a previous study.6 Therefore, it is plausible that the S protein of SARS-CoV-2 might mediate potent infectivity, even on cells expressing low hACE2, which would, in turn, explain why the transmission rate of SARS-CoV-2 is so high. It is also possible that other receptors mediate the entry of SARS-CoV-2 into T cells, such as CD147, present on the surface of T lymohocytes,8 which was recently reported to be a novel invasive route for SARS-CoV-2.9
Fig. 1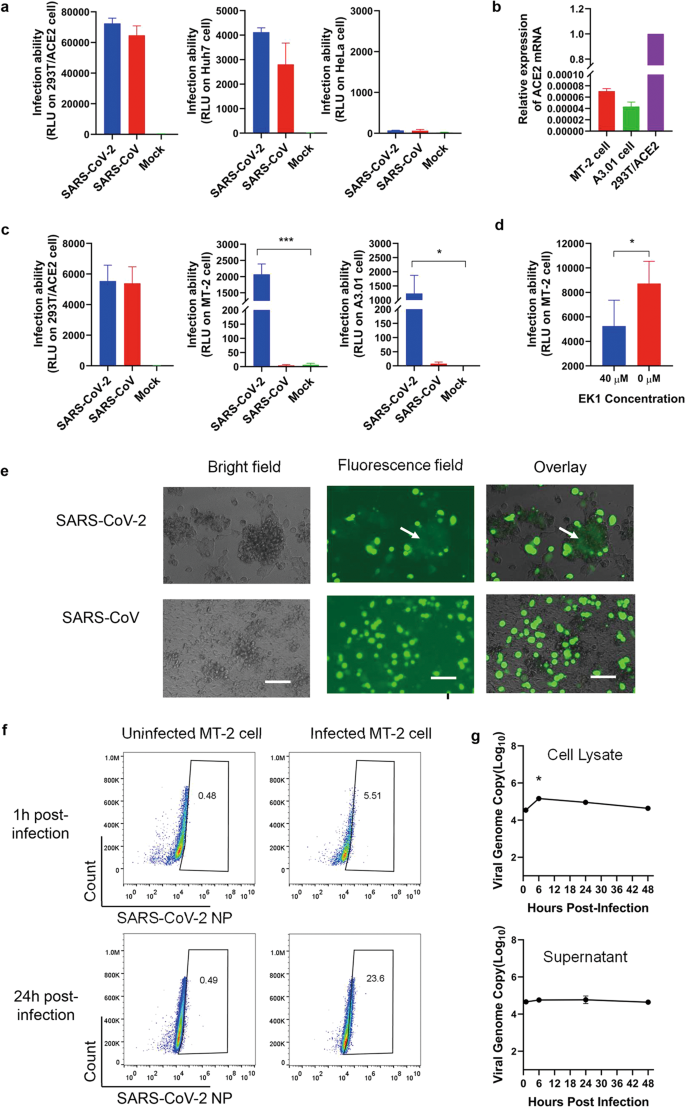
Sensitivity of T lymphocytes to SARS-CoV-2 infection. a Infection of pseudotyped SARS-CoV-2 and SARS-CoV. 293T/ACE2 and Huh7 are permissive cells, while HeLa is a nonpermissive cell line. b Expression of ACE2 mRNA in T cells. 293T/ACE2 cells used as a control. c Infection of pseudotyped SARS-CoV-2 and SARS-CoV on T lymphoid cell lines. d Inhibition of EK1 peptide on pseudotyped SARS-CoV-2 on MT-2 cells. e SARS-CoV-2 S-mediated cell–cell fusion on MT-2 cells. MT-2 cells were cocultured with 293T/SARS-CoV-2/EGFP cells. Cell–cell fusion was photographed under an optical microscope with fluorescence or visible light. Fused cells were indicated with a white arrow. Scale bars 800 μm. f Detection of SARS-CoV-2 NP-positive cells with flow cytometry. MT-2 cells were infected with SARS-CoV-2 at 1TCID50 per cell, respectively. Cells were fixed and permeabilized at 24 and 48 h post infection and immunolabeled for detection of SARS-CoV-2 NP. g Infection of MT-2 cells by SARS-CoV-2 is abortive. Cells were inoculated with SARS-CoV-2 at 1TCID50 per cell and harvested at 1, 6, 24, and 48 h post infection. Cell lysate (above) and supernatant (below) were collected to detect the viral N gene with RT-qPCR. In panels, bars and error bars represent means and standard deviations. Statistical analyses were performed using the unpaired t test. *p < 0.05
Full size image
To assess if SARS-CoV-2 enters T lymphocytes through non-receptor-mediated endocytosis, we used EK1 peptide which has been shown to inhibit SARS-CoV-2 spike protein (S) mediated cell–cell fusion and pseudovirus infection.7,10 Specifically, it inhibits receptor-mediated infection by interacting with HR1 to block the formation of the six-helix bundle (6-HB), further inhibiting fusion between viral and target cell membranes. We found that the EK1 peptide had significant inhibitory activity against SARS-CoV-2 pseudoviruses on MT-2 cells (Fig. 1d), suggesting that virus entry depends on receptor-mediated fusion. However, only a high concentration (40 μM) of EK1 had inhibitory activity on MT-2 cells. Meanwhile, the IC50 value of EK1 was 2.38 μM on 293T/ACE2 cells.10 These results suggest that SARS-CoV-2 can also enter T lymphocytes through the receptor-mediated endocytosis pathway. To clarify, we performed a SARS-CoV-2 S-mediated cell–cell fusion assay according to previous studies.7,10 After 48 h of coculture, 293T cells expressing SARS-CoV-2 S protein fused with MT-2 cells. Compared with unfused cells, the fused cells clustered together and appeared as a large faint green fluorescent mass. In contrast, no fused cells were found in the SARS-CoV coculture (Fig. 1e). Therefore, it can be concluded that SARS-CoV-2 might infect T cells through S protein-mediated membrane fusion.
To further determine the susceptibility of MT-2 cells to live virus, we used SARS-CoV-2 to infect MT-2 cells and detected the SARS-CoV-2 nucleoprotein (NP) in the cells as reported previously.6 Notably, several MT-2 cells were infected with SARS-CoV-2 (Fig. 1f). Quantitatively, the percentage of SARS-CoV-2 NP-positive MT-2 cells was 23.11% higher than that of uninfected cells at 24 h post infection, which is about 4.6-fold of the portion at 1 h (Fig. 1f). This result means that the virus penetrated MT-2 cells at 24 h and infected them.
Given that MERS-CoV can efficiently infect, but not replicate, in T lymphocytes,6 we further detected the number of viral genome copies at different time points post infection to explore the replication characteristics of SARS-CoV-2 in MT-2 cells. Similar to MERS-CoV, SARS-CoV-2 failed to replicate in MT-2 cells (Fig. 1g). The number of viral genome copies at 6 h was significantly higher than other time points in the cell lysate, but always remained steady at all time points in the supernatants. These results suggest that SARS-CoV-2 may enter MT-2 cells at 6 h post infection, but does not replicate, and then the viral RNA degrade. In supernatants, the detected viral copies might be the background of the residual virions, similar to the results of the previous MERS-CoV study (Fig. 1g).6
Based on the results of pseudovirus and live virus infection, here we proved that (1) SARS-CoV-2 could infect T cells, (2) SARS-CoV-2 infected T cells through receptor-dependent, S protein-mediated membrane fusion, and (3) infection could be inhibited by EK1 peptide. However, we observed a very low expression level of hACE2 in T cells; therefore, we further proposed that a novel receptor might mediate SARS-CoV-2 entry into T cells. Similar to MERS-CoV, SARS-CoV-2 infection of T cells is abortive. A recent study reported that viral reads barely displayed in PBMC samples from COVID-19 patients through transcriptome sequencing of RNAs. Thus, it was inferred that SARS-CoV-2 could not infect PBMCs. However, the transcriptomic characteristics of PBMCs were detected and analyzed from three patients. Two SARS-CoV-2 reads were detected in one patient’s PBMCs, and zero reads in another.3 This result could be attributed to nonproductive replication of SARS-CoV-2 in T lymphocytes, with little viral genome in PBMCs possibly degrading in the sample collection and RNA extraction process. Thus, the questions of SARS-CoV-2 infection and replication in primary T cells and whether the infection induces apoptosis in T cells still need further research, potentially evoking new ideas about pathogenic mechanisms and therapeutic interventions.
References- 1.
Jiang, S. et al. A distinct name is needed for the new coronavirus. Lancet https://doi.org/10.1016/S0140-6736(20)30419-0 (2020). - 2.
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). - 3.
Xiong, Y. et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. https://doi.org/10.2139/ssrn.3549993 (2020). - 4.
Zeng, Q. et al. Mortality of COVID-19 is Associated with Cellular Immune Function Compared to Immune Function in Chinese Han Population (2020). Preprint at https://doi.org/10.1101/2020.03.08.20031229 (2020). - 5.
Zheng, M. et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. https://doi.org/10.1038/s41423-020-0402-2 (2020). - 6.
Chu, H. et al. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 213, 904–914 (2016). - 7.
Xia, S. et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 5, eaav4580 (2019). - 8.
Koch, C. et al. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 11, 777–786 (1999). - 9.
Wang, K. et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. Preprint at https://doi.org/10.1101/2020.03.14.988345 (2020). - 10.
Xia, S. et al. Inhibition of SARS-CoV-2 infection (previously 2019-nCoV) by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. https://doi.org/10.1038/s41422-020-0305-x (2020).
Download references
Acknowledgements
We are very grateful to Qian Wang, Core Facility of Microbiology and Parasitology (SHMC), Fudan University. This work was supported by the National Megaprojects of China for Major Infectious Diseases (2018ZX10301403 to L.L.) and the National Natural Science Foundation of China (81822045 to L.L.; 81630090 to S.J.; 81703571 to W.X.).
Author information
Author notes- These authors contributed equally: Xinling Wang, Wei Xu, Gaowei Hu
- Key Laboratory of Medical Molecular Virology (MOE/NHC/CAMS), School of Basic Medical Sciences and Biosafety Level 3 Laboratory, Fudan University, Shanghai, China
- Xinling Wang
- , Wei Xu
- , Gaowei Hu
- , Shuai Xia
- , Zhiping Sun
- , Zezhong Liu
- , Youhua Xie
- , Rong Zhang
- , Shibo Jiang
- & Lu Lu
- Lindsley F. Kimball Research Institute, New York Blood Center, New York, NY, USA
- Shibo Jiang
Correspondence to Shibo Jiang or Lu Lu.
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Wang, X., Xu, W., Hu, G. et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol (2020). https://doi.org/10.1038/s41423-020-0424-9
Download citation- Received21 March 2020
- Accepted24 March 2020
- Published07 April 2020
- DOIhttps://doi.org/10.1038/s41423-020-0424-9
-
Can anyone explain how this relates to transmission rate? ACE2 is common in many cell types and if I understand this paper it can get into T cells but not replicate. I am confused as to which T cell they think it is infecting if it is CD8+ this could actually be helpful later in the disease but for CD4+ I assume it would be a problem. This is at the edge of my understanding so I would be most grateful if anyone with knowledge could help clarify the significance of this paper.Therefore, it is plausible that the S protein of SARS-CoV-2 might mediate potent infectivity, even on cells expressing low hACE2, which would, in turn, explain why the transmission rate of SARS-CoV-2 is so high.
Comment
-
My understanding is as follows:- If progenitor T cells are infected and destroyed then the ability of the body to counter the virus is greatly if not completely diminished, and that would lead to very high viral loads (increasing infectivity), and that this situation could subsequently persist for a long time as an effective antibody response would not be mounted by the host. instead there is a war of attrition between the virus and T cell production of all types. Death of infected T cells would also prevent B cell maturation and formation which in turn would lead to the extremely low antibody counts in some people, in a very short time frame after the infection. In other words it could be responsible for both Elisa tests failing, and increased susceptibility to either re-infection, or (if like HIV) it resides inside Tcells it could be the source of a resurgence of infection some time after recovery. There is also the possibility that with a second cell entry mechanism (CD147), other cell types could be becoming infected independently of ACE2 which may leave a viral reservoir elsewhere in the body that can become a persistent problem, again much like HIV.
Comment
-
I also remember reading a paper about a furin cleavage site for the S spike protein on SARS-CoV-2 , which as memory serves from H5N1 studies, may mean that it could potentially attack many more cell types I need to do some delving into the data to get my thoughts in order as we looked extensively at this then.. it was somehow linked to H5N1 potential virulence, but I am not sure what the relevance would be here yet.
Comment
-
Thanks, yes if it attacks the naive T-cells then CD8+, T-helper and NK would all be down regulated. TWiV 601 discussion touched on the poly-basic cleavage site but did not think it had an important role in SARS-2 where the TMPRSS2 protein is involved in enabling endocytosis. In flu the low path form, without a polybasic cleavage site, the HA can only be cleaved by a limited range of proteases found in the upper respiratory tract (Tryptase Clara is one but there may be others). With the poly-basic addition the cleavage site is easier to access and many more common host proteases can get at the cleavage site increasing tissue tropism as now any cell with the right Sialic acid residue becomes a potential target.Last edited by JJackson; April 15, 2020, 12:08 PM.
Comment
-
Does this form of attack on the bodies immune system also suppress the activation of monocytes and/or lymphocytes which would suppress the production of endogenous pyrogens which would suppress a body temperature increase? Would this also suppress a normal inflammatory response, thus reducing the lung impact and potentially achieving an infection equilibrium?
In other words, could this attack explain why some people are asymptomatic and worse imply they would also carry high viral loads and be super spreaders?
I'm asking because I don't have the expertise to answer this myself.
Comment
-
Just found this pre-print paper that summarises things far better than I can. Medical Countermeasures Analysis of 2019-nCoV and Vaccine Risks for Antibodydependent Enhancement (ADE) It goes through known effects on immune system for SARS-CoV-2, as well as reporting some original research. Basically short cuts for a vaccine are not advisable, but the background discussion is also useful here..
You have to download the pdf from this link https://www.preprints.org/manuscript/202003.0138/v1
Comment
-
MSCOX & Vibrant There is yet another new TWiV out 602 https://www.microbe.tv/twiv/twiv-602/ with Stanley Perlman who is the person I had hoped they would have on at some stage as he probably knows more about Corona virus than anyone else. ADE, T-cell infection, T-cell memory vs antibody generation and everything else gets covered. It should go a long way to answering all our questions. If you have not listened to them I would also recommend 597 and 591 for an understanding of the immunology plus Immune 29 https://www.microbe.tv/immune/immune-29/ and https://www.youtube.com/watch?v=28gIJ2rrN7I
I am afraid that is several hours of watching/listening but is the basis of what I know about SARS-2 and the immune response.
Comment
Comment