[Source: PLoS ONE, full page: (LINK). Edited.]
OPEN ACCESS PEER-REVIEWED / RESEARCH ARTICLE
A Detailed Epidemiological and Clinical Description of 6 Human Cases of Avian-Origin Influenza A (H7N9) Virus Infection in Shanghai
Jindong Shi, Juan Xie, Zebao He, Yunwen Hu, Yanchao He, Qihui Huang, Beizheng Leng, Wei He, Ying Sheng, Fangming Li, Yuanlin Song, Chunxue Bai, Yong Gu, Zhijun Jie
Abstract
Background
The world?s first reported patient infected with avian influenza H7N9 was treated at the Fifth People?s Hospital of Shanghai. Shortly thereafter, several other cases emerged in the local area. Here, we describe the detailed epidemiological and clinical data of 6 cases of avian influenza H7N9.
Methods and Findings
We analyzed the epidemiologic and clinical data from clustered patients infected with H7N9 in the Minhang District of Shanghai during a 2-week period. Of the 6 patients, 2 were from a single family. In addition, 3 patients had a history of contact with poultry; however, all 6 patients lived in the proximity of 2 food markets where the H7N9 virus was detected in chickens and pigeons. The main symptoms were fever, cough, and hemoptysis. At onset, a decreased lymphocyte count and elevated creatine kinase, lactate dehydrogenase, procalcitonin, and C-reactive protein levels were observed. As the disease progressed, most patients developed dyspnea and hypoxemia. Imaging studies revealed lung consolidation and multiple ground-glass opacities in the early stage, rapidly extending bilaterally. All patients were treated with oseltamivir tablets beginning on days 3?8 after onset. The main complications were as follows: acute respiratory distress syndrome (ARDS; 83.3%), secondary bacterial infection (66.7%), pleural effusion (50%), left ventricular failure (33.3%), neuropsychiatric symptoms (33.3%), and rhabdomyolysis (16.7%). Of the 6 patients, 4 died of ARDS, with 2 patients recovering from the infection.
Conclusions
An outbreak of H7N9 infection occurred in the Minhang District of Shanghai that easily progressed to acute respiratory distress syndrome. Two cases showed family aggregation, which led us to identify the H7N9 virus and indicated that human transmission may be involved in the spread of this infection.
_____
Citation: Shi J, Xie J, He Z, Hu Y, He Y, et al. (2013) A Detailed Epidemiological and Clinical Description of 6 Human Cases of Avian-Origin Influenza A (H7N9) Virus Infection in Shanghai. PLoS ONE 8(10): e77651. doi:10.1371/journal.pone.0077651
Editor: Kwok H. Chan, University of Hong Kong, Hong Kong
Received: May 31, 2013; Accepted: September 13, 2013; Published: October 15, 2013
Copyright: ? 2013 Shi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was partially funded by the grants from the China National Mega-projects for Infectious Diseases (2012ZX10004-211), Shanghai Committee of Science and Technology (134119b1200), and the training program of young doctors foundation of Shanghai Municipal Health Bureau (2012-105). The funders had no role in study design, data collection and analysis, decision to publish, or preparation and revision of the manuscript.
Competing interests: These authors have no support or funding to report.
_________
Introduction
Avian influenza is an infectious poultry disease caused by avian influenza A viruses, which are classified as low pathogenic avian influenza A (LPAI) and high pathogenic avian influenza A (HPAI) viruses. LPAI viruses rarely cause severe avian diseases, whereas HPAI viruses can cause deadly infectious diseases in birds, spreads rapidly, and can infect humans. Almost all HPAI viruses belong to the H5, H7, and H9 subtypes. The main subtypes of avian influenza viruses known to infect humans are H7, H5, H9, and H10 and N1, N2, N3, and N7. There have been over 100 documented cases of humans infected with H7 viruses from poultry or wildfowl mainly in the United States, United Kingdom, Italy, the Netherlands, Canada, and other European countries [1-6], while N9-subtype viruses had never been reported in humans until recently. Most human infections with avian influenza A (H7) viruses exhibit mild symptoms or present as latent infections. Most cases result in conjunctivitis and symptoms of respiratory tract infection that are mild to moderate in severity, with only one fatal case reported in a patient with H7N7 virus infection [7]. From 1999 to 2003, Puzelli found that 7 of 185 poultry workers (3.8%) were positive for specific antibodies to avian influenza A H7 viruses, of which only 1 case resulted in conjunctivitis, whereas the remaining 6 cases exhibited no clinical symptoms [4]. By May 28, 2013, 123 patients were diagnosed with laboratory-confirmed H7N9 virus infections and admitted to the hospital, and 37 (30%) patients died of the infection; this outcome is inconsistent with those of previous reports on avian influenza A H7 virus infections [8].
In February 2013, the first ever case of human infection with H7N9 was encountered at the respiratory department of the Fifth People?s Hospital of Shanghai [9]. During the following 2-week period, the hospital received another 5 patients with H7N9 infection. All cases were localized to the Minhang District of Shanghai, and the disease onset in all cases occurred within a 2-week period. Of the 6 patients, 2 emerged from a single family, suggesting that aggregation exists to some extent. In addition, H7N9 avian influenza and H5N1, both of which are fatal, show similar clinical symptoms and disease progression [10,11]. Therefore, early diagnosis and treatment, with an improved cure rate are important to control these infections [11]. Here, we summarize the findings of 6 cases of pneumonia caused by H7N9 infection.
Methods
Ethics statement
This work was approved by the Human Research Committee at the Fifth people`s hospital of Shanghai and Shanghai Public Health Clinical Center, and written informed consent was obtained from the patients or their families.
Subjects
All confirmed cases of H7N9 referred to the Fifth People?s Hospital of Shanghai during the period from February to March 2013 were enrolled in this study. H7N9 infection was confirmed by the Public Health Clinical Center Affiliated with Fudan University as well as the Chinese Center for Disease Control and Prevention.
Collection of clinical data and laboratory specimens
General patient information, clinical signs and symptoms, laboratory test results, chest radiographic findings, and treatment were recorded.
Information regarding the patients? recent activities and environment was also analyzed. Clinical signs and laboratory testing results refer to testing that was performed before the patients were hospitalized or within 2 days of hospitalization.
Viral detection
Throat-swab, sputum, and blood specimens obtained from all patients with suspected H7N9 infection were sent to the Shanghai Public Health Clinical Center. A specific reverse transcriptase-polymerase chain reaction (RT-PCR) assay for avian influenza H7N9 was used to detect the virus in throat-swab and sputum specimens. In addition, H7N9 virus was also isolated from throat-swab and sputum specimens. Serum samples from the 2 surviving patients obtained at both the acute phase and the recovery stage were tested for specific antibodies to H7N9 virus.
Results
Demographic characteristics
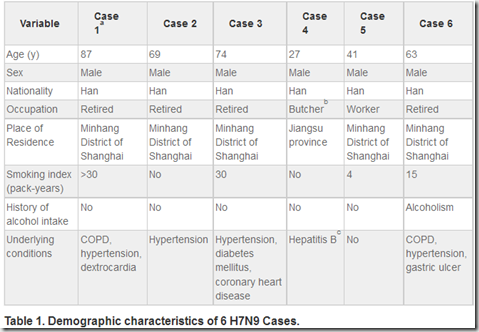
All 6 patents were male, Han nationality, aged from 27 to 87 years old, 4 were retired individuals, 1 in-service worker, and 1 pork peddler. Four patients had history of tobacco, 1 had history of drinking, and 5 had at least 1 of the following underlying diseases: chronic obstructive pulmonary diseases, hypertension, dextrocardia, diabetes, coronary heart disease, hepatitis B, and/or gastric ulcer. (Table 1)
(?)
The time course of case identification, treatment, and diagnosis
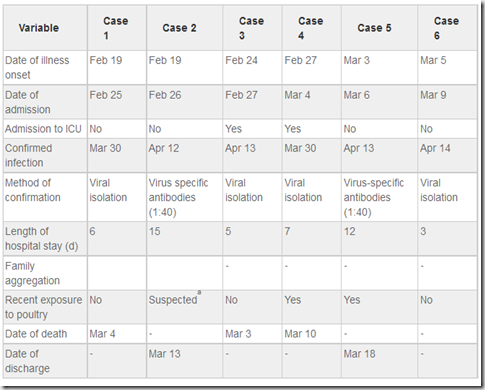
Disease onset in the 6 cases occurred within a 2-week period from 19 February and 5 March 2013. The time range from onset to hospitalization was 3?7 days (mean, 4.6 days). Cases 3 and 4 were admitted to the intensive care unit (ICU) because of disease progression on the first and second day of hospitalization, respectively. The length of hospital stay was 3?15 days (mean, 8 days). H7N9 was confirmed by RT-PCR and virus isolation in 4 cases and by elevated (4? that of normal) levels of specific antibodies to H7N9 in the acute phase and recovery stage in 2 cases. Case 2 was the son of case 1, whose other son (age, 55 years) developed severe pneumonia on February 11 and died on February 28. However, the H7N9 virus was not detected in respiratory specimens from the deceased son by RT-PCR or viral isolation. (Table 2)
(?)
History of exposure to birds and residence state
Among the 6 patients, 5 were residents of the Minhang District of Shanghai and had not left Shanghai prior to the onset of illness. One patient, who was a pork seller at a market in the Minhang District, was originally from the Jingsu province and had been a resident of Minhang District for 9 months at the time of disease onset. All 6 patients lived in the proximity of 2 food markets where poultry were traded and H7N9 virus carrier birds had been discovered. Trading of poultry was banned in the markets on April 4. Two patients had a history of exposure to live birds and 1 had a history of suspected exposure. (Figure 1)
(?)
Medical observation of the exposed population
There were 122 people who were in close contact with the 6 infected patients, mainly consisting of family members, relatives, and medical staff. All close contacts were kept under medical observation for 1 week, and clinical signs and symptoms similar to those of the patients were not observed.
Signs and symptoms
All patients had fever (100%), with 5 (83.3%) patients developing high fever (temperature, 38.6?41?C) and 1 patient with chills and shivering. All patients had cough, 4 (66.7%) of whom had white sputum, 1 had yellow sputum, and 5 had hemoptysis. Five patients (83.3%) had dyspnea. One patient (16.7%) had evident chest pain. Two patients had nausea and vomiting. Patient symptoms are shown in Table 3.
(?)
Laboratory findings
Blood cell count and biochemical test results were obtained prior to admission or within 2 days of admission. White blood cell (WBC) counts were normal or below normal levels. In particular, the lymphocyte and platelet counts were low in 4 critical patients. In addition, all 6 patients had elevated aspartate aminotransferase levels, 5 of whom had elevated creatine kinase (CK) and lactate dehydrogenase (LDH) levels; these laboratory results were missing for Case 5. The C-reactive protein (CRP) levels of all patients were low, and 5 patients had elevated β-natriuretic peptide levels, which was not tested in case 5. Blood gas analysis suggested that 5 patients had hypoxemia, and 4 patients had decreased PCO<SUB>2</SUB> and elevated pH values as a result of hyperventilation. Laboratory findings are shown in Table 4.
Imaging features
Chest radiography and lung computed tomography suggested that consolidation and multiple ground-glass opacities emerged in the early stage of disease and rapidly extended bilaterally. In 2 of 6 cases, pulmonary symptoms such as acute respiratory distress syndrome (ARDS) were detected. Furthermore, minor pleural effusion and subcutaneous and mediastinal emphysema were observed (Figure 2).
Treatment, complications, and outcome
Oseltamivir (tablet, 75 mg bid) therapy was initiated 3?8 days after disease onset in all patients. One recovering patient was treated with a combination of oseltamivir (tablet, 75 mg bid) and amantadine (tablet, 0.2, bid). Broad-spectrum antibiotics against Gram-positive and Gram-negative bacteria and atypical pathogens, such as penicillium, carbon alkene, and fluoroquinolone, were administered to all patients. Except for the recovering patient, all patients were treated with intravenous glucocorticoid as an anti-inflammatory at a dose of 80?240 mg/d. Intravenous immunoglobulin was administered in 4 patients, and thymosin was administered in 2 patients.
In terms of complications, 4 patients developed ARDS between days 3 and 9 (mean, day 6) after disease onset. Three patients had secondary bacterial infections, 3 had pleural effusion, 2 had left heart functional failure, 2 had neuropsychiatric symptoms, and 1 had rhabdomyolysis. Among the 2 recovering patients, 1 patient had no complications, while the other developed secondary bacterial infections. (Table 5)
Discussion
H7N9 is a subtype of the avian influenza A virus. Until 2013, 25 types of H7N9 viruses had been detected in birds worldwide. Previously detected H7N9 viruses only infected birds, and these infections were generally mild. However, animal experiments showed that both HPAI H7N3 and LPAI H7N9 could effectively replicate in mice without adaptation, as well as in the upper and lower respiratory tract of ferrets. HPAI H7N3 was also found to be lethal in mice and showed transmission through contact in the ferret model, whereas LPAI H7N9 could not be transmitted in the ferret model [12-15]. In March 2013, 3 cases of human H7N9 avian influenza infection were reported in Shanghai and Anhui, China. Research showed that the H7N9 virus reported at this time was a novel, reassortant influenza A virus [9]. The virus contained 8 gene segments, with only the NA gene being closely related to that of another H7N9 virus (KO14). The HA gene was similar to that of an H7N3 virus (ZJ12) from a nearby region (Zhejiang province) in China. All the internal gene segments were closely related to those from other avian H9N2 viruses.
The sources of avian influenza infection are mainly poultry or wild birds, which are carriers or sufferers of the disease. However, humans can be infected with avian influenza viruses via several transmission routes such as through direct avian-to-human contact, which is the most common mode of transmission; environment-to-human transmission; and mother-to-child vertical transmission [16-19]. Our study showed that 6 cases of pneumonia caused by H7N9 were detected in the area with close proximity to the Fifth People?s Hospital of Shanghai within a 2 week-period from 19 February and 5 March 2013. Among the 6 patients, 2 were related (father and son); in addition, several days prior to disease onset, the younger son (age, 55 years) developed high fever, cough, dyspnea, and rapidly developed ARDS, which was similar to the disease course of the other 2 family members who had confirmed cases of H7N9 infection. However, the H7N9 virus was not detected in his blood and respiratory specimens.
The 6 patients lived in the proximity of the 2 markets where live birds were traded, and 2 patients had a history of exposure to birds; 1 had a history of suspected exposure to birds, and 1 patient worked in one of the markets. After the outbreak, the health department identified H7N9 viruses in carrier poultry in the 2 markets. Live bird trading was then forbidden. Thereafter, no additional cases of H7N9 infection were reported in the Minhang District. The above evidence suggests that the outbreak of infection caused by H7N9 viruses in the district was likely because of live bird carriers of H7N9 viruses in the 2 markets. The patients of this study had close contact with 122 people, who were mainly family members, relatives, and medical staff.
These individuals were kept under medical observation for 1 week, and similar signs and symptoms to those of the patients were not observed. The lack of clinical infection signs in the contact individuals suggests that the efficiency of human-to-human transmission is low. Even without appropriate quarantine measures, the possibility of hospital infection is small. Therefore, a similar exposure history among direct relatives is believed to be the cause of clustering cases in a family.
A general lack of immunity to avian influenza viruses leads to human infection, which can result in severe outcomes. Previously detected LPAI H7 avian influenza viruses were reported to cause mild symptoms, mainly conjunctivitis or flu-like symptoms, in humans [1,6,20,21], although H7N7 avian influenza viruses have been reported to also cause more serious complications such as pneumonia and ARDS [21,22]. For example, in the 2003 outbreak of the H7N7 virus in the Netherlands, 82 of the 89 cases (92.1%) manifested as conjunctivitis, whereas the remaining patients presented with an influenza-like illness [5]. HPAI H5N1 avian influenza viruses have caused more severe illnesses, from asymptomatic infection to fatal pneumonia and multiple organ failure than the H7 subtype. The characteristics of H5N1 avian influenza virus infection include a high case rate, high fatality rate, and development of shock in the early stages of disease [23,24]. The 6 patients infected with H7N9 viruses in this report showed signs and symptoms similar to those of patients infected with H5N1 viruses; all infected patients had pneumonia, with most rapidly progressing to ARDS. In the initial stage of disease, patients presented with fever and bloody sputum accompanied by chills, chest pain, nausea, and vomiting in some of the patients. Dyspnea developed 3?7 days after disease onset. Upper respiratory tract infection symptoms such as nasal congestion, runny nose, and sore throat, were not observed in any case, which was probably because of the lack of early diagnosis in patients with mild symptoms.
Laboratory testing in the early stage showed that the WBC count was normal or lower than normal; the lymphocyte and platelet counts were low; and serum CRP, procalcitonin, CK, and LDH levels were elevated. Blood gas analysis also suggested hypoxemia and different degrees of excessive ventilation. Chest imaging suggested that consolidation and multiple ground-glass opacities of the lung developed in the early stage of the disease, and rapidly extended bilaterally. Patients with severe disease also showed ARDS-like symptoms. Furthermore, minor pleural effusion as well as subcutaneous and mediastinal emphysema were observed in some patients with severe disease. The above evidence suggests that fever, cough, hemoptysis, normal or low WBC levels, low lymphocyte counts, elevated CK and LDH levels, and pulmonary exudative lesions are significant characteristics of H7N9 infection.
Within 7 days of disease onset, H7N9 infection was confirmed in 4 of the 6 cases by RT-PCR assay and viral isolation using respiratory samples. The remaining 2 cases were confirmed using a specific antibody to the H7N9 virus in double serum samples from the acute and recovery stages. The results suggest that human infection with H7N9 viruses can be diagnosed early by RT-PCR of respiratory samples such as throat-swabs and sputum specimens, and by viral isolation from these samples. After admission, all patients were treated with broad-spectrum antibiotics, which were not effective in resolving symptoms. On days 3?8, all patients were treated with oral oseltamivir, and severely ill patients were treated with intravenous glucocorticoid, immunoglobulin, and thymosin. Several complications were observed, including ARDS, secondary bacterial infection, pleural effusion, left heart functional failure, neuropsychiatric symptoms, and rhabdomyolysis. ARDS developed on days 3?9 (mean, 6 days), the time course of which is consistent with that of reported cases of H5N1 infection in Thailand [25].
Factors affecting the prognosis of H7N9 viruses are unknown. Case 2 was a 69-year-old man with a history of hypertension and no history of drinking, and he recovered from the infection. Case 5 was a 41-year-old man with a history of smoking (smoking index, 4 pack-years) and no history of drinking or underlying disease, who also recovered. The ages of the other 4 patients were 87, 74, 27, and 63 years, and each had at least 1 underlying disease and a history of smoking and drinking; these patients ultimately died of the infection. These findings suggest that age may not be related to prognosis; however, underlying disease does appear to impact prognosis.
The patients who recovered had no dyspnea, whereas those who died developed different degrees of dyspnea. In terms of laboratory testing, compared to the patients who died, the patients who recovered had only slightly varying platelet counts and CK and PO<SUB>2</SUB> levels. In terms of complications, 1 patient who recovered had no complications, whereas the other developed secondary bacterial infections. In contrast, patients who died had 2?4 complications each. The time to treatment with oseltamivir therapy in the recovered cases was days 7 and 8, whereas it was days 3?8 in the patients who died. The above evidence implies that smoking, drinking, and underlying diseases may be related to the prognosis of H7N9 infection; dyspnea, decreased platelet counts, elevated CK levels, and hypoxemia may be associated with poor prognosis. The time to initiation of antiviral therapy seems to have no impact on prognosis, whereas the presence of complications may be associated with poor prognosis [10].
In conclusion, the first ever patient infected with H7N9 was treated at the Fifth People?s Hospital of Shanghai in February 2013. Within a 2-week period, several other cases of H7N9 infection emerged around 2 markets near the hospital. Fever, cough, sputum with blood, low lymphocyte counts, elevated CK and LDH levels, and pulmonary exudative lesions are significant characteristics of H7N9 infection, which easily progresses to ARDS. Among the cases, there was family clustering, which led to a high suspicion of contagious respiratory virus infection. In the early stage, human infection with H7N9 can be diagnosed by RT-PCR and viral isolation from respiratory specimens. Smoking, drinking, underlying diseases, dyspnea, low platelet counts, elevated CK levels, hypoxemia, and complications may be related to poor prognosis. However, diagnosis and treatment may be delayed because of the limited experience with this infection and small number of cases, which were among the first cases of H7N9 infection to be identified. Future studies are needed to elucidate the pathogenicity, transmissibility, and clinical features of H7N9 infection. In addition, techniques for early diagnosis to enable early administration of antiviral therapy and determination of the the factors affecting prognosis require further investigation.
Acknowledgments
The authors would like to thank the Fifth People?s Hospital of Shanghai and Shanghai Public Health Clinical Center, Fudan University for providing medical resources.
Author Contributions
Conceived and designed the experiments: ZJ YG. Performed the experiments: JS JX ZH YWH YCH QH BL WH YS FL. Analyzed the data: JS YCH ZJ. Contributed reagents/materials/analysis tools: JS JX ZH YWH YCH QH BL WH YS FL YLS CB. Wrote the manuscript: JS ZJ.
References
-
------
OPEN ACCESS PEER-REVIEWED / RESEARCH ARTICLE
A Detailed Epidemiological and Clinical Description of 6 Human Cases of Avian-Origin Influenza A (H7N9) Virus Infection in Shanghai
Jindong Shi, Juan Xie, Zebao He, Yunwen Hu, Yanchao He, Qihui Huang, Beizheng Leng, Wei He, Ying Sheng, Fangming Li, Yuanlin Song, Chunxue Bai, Yong Gu, Zhijun Jie
Abstract
Background
The world?s first reported patient infected with avian influenza H7N9 was treated at the Fifth People?s Hospital of Shanghai. Shortly thereafter, several other cases emerged in the local area. Here, we describe the detailed epidemiological and clinical data of 6 cases of avian influenza H7N9.
Methods and Findings
We analyzed the epidemiologic and clinical data from clustered patients infected with H7N9 in the Minhang District of Shanghai during a 2-week period. Of the 6 patients, 2 were from a single family. In addition, 3 patients had a history of contact with poultry; however, all 6 patients lived in the proximity of 2 food markets where the H7N9 virus was detected in chickens and pigeons. The main symptoms were fever, cough, and hemoptysis. At onset, a decreased lymphocyte count and elevated creatine kinase, lactate dehydrogenase, procalcitonin, and C-reactive protein levels were observed. As the disease progressed, most patients developed dyspnea and hypoxemia. Imaging studies revealed lung consolidation and multiple ground-glass opacities in the early stage, rapidly extending bilaterally. All patients were treated with oseltamivir tablets beginning on days 3?8 after onset. The main complications were as follows: acute respiratory distress syndrome (ARDS; 83.3%), secondary bacterial infection (66.7%), pleural effusion (50%), left ventricular failure (33.3%), neuropsychiatric symptoms (33.3%), and rhabdomyolysis (16.7%). Of the 6 patients, 4 died of ARDS, with 2 patients recovering from the infection.
Conclusions
An outbreak of H7N9 infection occurred in the Minhang District of Shanghai that easily progressed to acute respiratory distress syndrome. Two cases showed family aggregation, which led us to identify the H7N9 virus and indicated that human transmission may be involved in the spread of this infection.
_____
Citation: Shi J, Xie J, He Z, Hu Y, He Y, et al. (2013) A Detailed Epidemiological and Clinical Description of 6 Human Cases of Avian-Origin Influenza A (H7N9) Virus Infection in Shanghai. PLoS ONE 8(10): e77651. doi:10.1371/journal.pone.0077651
Editor: Kwok H. Chan, University of Hong Kong, Hong Kong
Received: May 31, 2013; Accepted: September 13, 2013; Published: October 15, 2013
Copyright: ? 2013 Shi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was partially funded by the grants from the China National Mega-projects for Infectious Diseases (2012ZX10004-211), Shanghai Committee of Science and Technology (134119b1200), and the training program of young doctors foundation of Shanghai Municipal Health Bureau (2012-105). The funders had no role in study design, data collection and analysis, decision to publish, or preparation and revision of the manuscript.
Competing interests: These authors have no support or funding to report.
_________
Introduction
Avian influenza is an infectious poultry disease caused by avian influenza A viruses, which are classified as low pathogenic avian influenza A (LPAI) and high pathogenic avian influenza A (HPAI) viruses. LPAI viruses rarely cause severe avian diseases, whereas HPAI viruses can cause deadly infectious diseases in birds, spreads rapidly, and can infect humans. Almost all HPAI viruses belong to the H5, H7, and H9 subtypes. The main subtypes of avian influenza viruses known to infect humans are H7, H5, H9, and H10 and N1, N2, N3, and N7. There have been over 100 documented cases of humans infected with H7 viruses from poultry or wildfowl mainly in the United States, United Kingdom, Italy, the Netherlands, Canada, and other European countries [1-6], while N9-subtype viruses had never been reported in humans until recently. Most human infections with avian influenza A (H7) viruses exhibit mild symptoms or present as latent infections. Most cases result in conjunctivitis and symptoms of respiratory tract infection that are mild to moderate in severity, with only one fatal case reported in a patient with H7N7 virus infection [7]. From 1999 to 2003, Puzelli found that 7 of 185 poultry workers (3.8%) were positive for specific antibodies to avian influenza A H7 viruses, of which only 1 case resulted in conjunctivitis, whereas the remaining 6 cases exhibited no clinical symptoms [4]. By May 28, 2013, 123 patients were diagnosed with laboratory-confirmed H7N9 virus infections and admitted to the hospital, and 37 (30%) patients died of the infection; this outcome is inconsistent with those of previous reports on avian influenza A H7 virus infections [8].
In February 2013, the first ever case of human infection with H7N9 was encountered at the respiratory department of the Fifth People?s Hospital of Shanghai [9]. During the following 2-week period, the hospital received another 5 patients with H7N9 infection. All cases were localized to the Minhang District of Shanghai, and the disease onset in all cases occurred within a 2-week period. Of the 6 patients, 2 emerged from a single family, suggesting that aggregation exists to some extent. In addition, H7N9 avian influenza and H5N1, both of which are fatal, show similar clinical symptoms and disease progression [10,11]. Therefore, early diagnosis and treatment, with an improved cure rate are important to control these infections [11]. Here, we summarize the findings of 6 cases of pneumonia caused by H7N9 infection.
Methods
Ethics statement
This work was approved by the Human Research Committee at the Fifth people`s hospital of Shanghai and Shanghai Public Health Clinical Center, and written informed consent was obtained from the patients or their families.
Subjects
All confirmed cases of H7N9 referred to the Fifth People?s Hospital of Shanghai during the period from February to March 2013 were enrolled in this study. H7N9 infection was confirmed by the Public Health Clinical Center Affiliated with Fudan University as well as the Chinese Center for Disease Control and Prevention.
Collection of clinical data and laboratory specimens
General patient information, clinical signs and symptoms, laboratory test results, chest radiographic findings, and treatment were recorded.
Information regarding the patients? recent activities and environment was also analyzed. Clinical signs and laboratory testing results refer to testing that was performed before the patients were hospitalized or within 2 days of hospitalization.
Viral detection
Throat-swab, sputum, and blood specimens obtained from all patients with suspected H7N9 infection were sent to the Shanghai Public Health Clinical Center. A specific reverse transcriptase-polymerase chain reaction (RT-PCR) assay for avian influenza H7N9 was used to detect the virus in throat-swab and sputum specimens. In addition, H7N9 virus was also isolated from throat-swab and sputum specimens. Serum samples from the 2 surviving patients obtained at both the acute phase and the recovery stage were tested for specific antibodies to H7N9 virus.
Results
Demographic characteristics
All 6 patents were male, Han nationality, aged from 27 to 87 years old, 4 were retired individuals, 1 in-service worker, and 1 pork peddler. Four patients had history of tobacco, 1 had history of drinking, and 5 had at least 1 of the following underlying diseases: chronic obstructive pulmonary diseases, hypertension, dextrocardia, diabetes, coronary heart disease, hepatitis B, and/or gastric ulcer. (Table 1)
(?)
The time course of case identification, treatment, and diagnosis
Disease onset in the 6 cases occurred within a 2-week period from 19 February and 5 March 2013. The time range from onset to hospitalization was 3?7 days (mean, 4.6 days). Cases 3 and 4 were admitted to the intensive care unit (ICU) because of disease progression on the first and second day of hospitalization, respectively. The length of hospital stay was 3?15 days (mean, 8 days). H7N9 was confirmed by RT-PCR and virus isolation in 4 cases and by elevated (4? that of normal) levels of specific antibodies to H7N9 in the acute phase and recovery stage in 2 cases. Case 2 was the son of case 1, whose other son (age, 55 years) developed severe pneumonia on February 11 and died on February 28. However, the H7N9 virus was not detected in respiratory specimens from the deceased son by RT-PCR or viral isolation. (Table 2)
(?)
History of exposure to birds and residence state
Among the 6 patients, 5 were residents of the Minhang District of Shanghai and had not left Shanghai prior to the onset of illness. One patient, who was a pork seller at a market in the Minhang District, was originally from the Jingsu province and had been a resident of Minhang District for 9 months at the time of disease onset. All 6 patients lived in the proximity of 2 food markets where poultry were traded and H7N9 virus carrier birds had been discovered. Trading of poultry was banned in the markets on April 4. Two patients had a history of exposure to live birds and 1 had a history of suspected exposure. (Figure 1)
(?)
Medical observation of the exposed population
There were 122 people who were in close contact with the 6 infected patients, mainly consisting of family members, relatives, and medical staff. All close contacts were kept under medical observation for 1 week, and clinical signs and symptoms similar to those of the patients were not observed.
Signs and symptoms
All patients had fever (100%), with 5 (83.3%) patients developing high fever (temperature, 38.6?41?C) and 1 patient with chills and shivering. All patients had cough, 4 (66.7%) of whom had white sputum, 1 had yellow sputum, and 5 had hemoptysis. Five patients (83.3%) had dyspnea. One patient (16.7%) had evident chest pain. Two patients had nausea and vomiting. Patient symptoms are shown in Table 3.
(?)
Laboratory findings
Blood cell count and biochemical test results were obtained prior to admission or within 2 days of admission. White blood cell (WBC) counts were normal or below normal levels. In particular, the lymphocyte and platelet counts were low in 4 critical patients. In addition, all 6 patients had elevated aspartate aminotransferase levels, 5 of whom had elevated creatine kinase (CK) and lactate dehydrogenase (LDH) levels; these laboratory results were missing for Case 5. The C-reactive protein (CRP) levels of all patients were low, and 5 patients had elevated β-natriuretic peptide levels, which was not tested in case 5. Blood gas analysis suggested that 5 patients had hypoxemia, and 4 patients had decreased PCO<SUB>2</SUB> and elevated pH values as a result of hyperventilation. Laboratory findings are shown in Table 4.
Imaging features
Chest radiography and lung computed tomography suggested that consolidation and multiple ground-glass opacities emerged in the early stage of disease and rapidly extended bilaterally. In 2 of 6 cases, pulmonary symptoms such as acute respiratory distress syndrome (ARDS) were detected. Furthermore, minor pleural effusion and subcutaneous and mediastinal emphysema were observed (Figure 2).
Treatment, complications, and outcome
Oseltamivir (tablet, 75 mg bid) therapy was initiated 3?8 days after disease onset in all patients. One recovering patient was treated with a combination of oseltamivir (tablet, 75 mg bid) and amantadine (tablet, 0.2, bid). Broad-spectrum antibiotics against Gram-positive and Gram-negative bacteria and atypical pathogens, such as penicillium, carbon alkene, and fluoroquinolone, were administered to all patients. Except for the recovering patient, all patients were treated with intravenous glucocorticoid as an anti-inflammatory at a dose of 80?240 mg/d. Intravenous immunoglobulin was administered in 4 patients, and thymosin was administered in 2 patients.
In terms of complications, 4 patients developed ARDS between days 3 and 9 (mean, day 6) after disease onset. Three patients had secondary bacterial infections, 3 had pleural effusion, 2 had left heart functional failure, 2 had neuropsychiatric symptoms, and 1 had rhabdomyolysis. Among the 2 recovering patients, 1 patient had no complications, while the other developed secondary bacterial infections. (Table 5)
Discussion
H7N9 is a subtype of the avian influenza A virus. Until 2013, 25 types of H7N9 viruses had been detected in birds worldwide. Previously detected H7N9 viruses only infected birds, and these infections were generally mild. However, animal experiments showed that both HPAI H7N3 and LPAI H7N9 could effectively replicate in mice without adaptation, as well as in the upper and lower respiratory tract of ferrets. HPAI H7N3 was also found to be lethal in mice and showed transmission through contact in the ferret model, whereas LPAI H7N9 could not be transmitted in the ferret model [12-15]. In March 2013, 3 cases of human H7N9 avian influenza infection were reported in Shanghai and Anhui, China. Research showed that the H7N9 virus reported at this time was a novel, reassortant influenza A virus [9]. The virus contained 8 gene segments, with only the NA gene being closely related to that of another H7N9 virus (KO14). The HA gene was similar to that of an H7N3 virus (ZJ12) from a nearby region (Zhejiang province) in China. All the internal gene segments were closely related to those from other avian H9N2 viruses.
The sources of avian influenza infection are mainly poultry or wild birds, which are carriers or sufferers of the disease. However, humans can be infected with avian influenza viruses via several transmission routes such as through direct avian-to-human contact, which is the most common mode of transmission; environment-to-human transmission; and mother-to-child vertical transmission [16-19]. Our study showed that 6 cases of pneumonia caused by H7N9 were detected in the area with close proximity to the Fifth People?s Hospital of Shanghai within a 2 week-period from 19 February and 5 March 2013. Among the 6 patients, 2 were related (father and son); in addition, several days prior to disease onset, the younger son (age, 55 years) developed high fever, cough, dyspnea, and rapidly developed ARDS, which was similar to the disease course of the other 2 family members who had confirmed cases of H7N9 infection. However, the H7N9 virus was not detected in his blood and respiratory specimens.
The 6 patients lived in the proximity of the 2 markets where live birds were traded, and 2 patients had a history of exposure to birds; 1 had a history of suspected exposure to birds, and 1 patient worked in one of the markets. After the outbreak, the health department identified H7N9 viruses in carrier poultry in the 2 markets. Live bird trading was then forbidden. Thereafter, no additional cases of H7N9 infection were reported in the Minhang District. The above evidence suggests that the outbreak of infection caused by H7N9 viruses in the district was likely because of live bird carriers of H7N9 viruses in the 2 markets. The patients of this study had close contact with 122 people, who were mainly family members, relatives, and medical staff.
These individuals were kept under medical observation for 1 week, and similar signs and symptoms to those of the patients were not observed. The lack of clinical infection signs in the contact individuals suggests that the efficiency of human-to-human transmission is low. Even without appropriate quarantine measures, the possibility of hospital infection is small. Therefore, a similar exposure history among direct relatives is believed to be the cause of clustering cases in a family.
A general lack of immunity to avian influenza viruses leads to human infection, which can result in severe outcomes. Previously detected LPAI H7 avian influenza viruses were reported to cause mild symptoms, mainly conjunctivitis or flu-like symptoms, in humans [1,6,20,21], although H7N7 avian influenza viruses have been reported to also cause more serious complications such as pneumonia and ARDS [21,22]. For example, in the 2003 outbreak of the H7N7 virus in the Netherlands, 82 of the 89 cases (92.1%) manifested as conjunctivitis, whereas the remaining patients presented with an influenza-like illness [5]. HPAI H5N1 avian influenza viruses have caused more severe illnesses, from asymptomatic infection to fatal pneumonia and multiple organ failure than the H7 subtype. The characteristics of H5N1 avian influenza virus infection include a high case rate, high fatality rate, and development of shock in the early stages of disease [23,24]. The 6 patients infected with H7N9 viruses in this report showed signs and symptoms similar to those of patients infected with H5N1 viruses; all infected patients had pneumonia, with most rapidly progressing to ARDS. In the initial stage of disease, patients presented with fever and bloody sputum accompanied by chills, chest pain, nausea, and vomiting in some of the patients. Dyspnea developed 3?7 days after disease onset. Upper respiratory tract infection symptoms such as nasal congestion, runny nose, and sore throat, were not observed in any case, which was probably because of the lack of early diagnosis in patients with mild symptoms.
Laboratory testing in the early stage showed that the WBC count was normal or lower than normal; the lymphocyte and platelet counts were low; and serum CRP, procalcitonin, CK, and LDH levels were elevated. Blood gas analysis also suggested hypoxemia and different degrees of excessive ventilation. Chest imaging suggested that consolidation and multiple ground-glass opacities of the lung developed in the early stage of the disease, and rapidly extended bilaterally. Patients with severe disease also showed ARDS-like symptoms. Furthermore, minor pleural effusion as well as subcutaneous and mediastinal emphysema were observed in some patients with severe disease. The above evidence suggests that fever, cough, hemoptysis, normal or low WBC levels, low lymphocyte counts, elevated CK and LDH levels, and pulmonary exudative lesions are significant characteristics of H7N9 infection.
Within 7 days of disease onset, H7N9 infection was confirmed in 4 of the 6 cases by RT-PCR assay and viral isolation using respiratory samples. The remaining 2 cases were confirmed using a specific antibody to the H7N9 virus in double serum samples from the acute and recovery stages. The results suggest that human infection with H7N9 viruses can be diagnosed early by RT-PCR of respiratory samples such as throat-swabs and sputum specimens, and by viral isolation from these samples. After admission, all patients were treated with broad-spectrum antibiotics, which were not effective in resolving symptoms. On days 3?8, all patients were treated with oral oseltamivir, and severely ill patients were treated with intravenous glucocorticoid, immunoglobulin, and thymosin. Several complications were observed, including ARDS, secondary bacterial infection, pleural effusion, left heart functional failure, neuropsychiatric symptoms, and rhabdomyolysis. ARDS developed on days 3?9 (mean, 6 days), the time course of which is consistent with that of reported cases of H5N1 infection in Thailand [25].
Factors affecting the prognosis of H7N9 viruses are unknown. Case 2 was a 69-year-old man with a history of hypertension and no history of drinking, and he recovered from the infection. Case 5 was a 41-year-old man with a history of smoking (smoking index, 4 pack-years) and no history of drinking or underlying disease, who also recovered. The ages of the other 4 patients were 87, 74, 27, and 63 years, and each had at least 1 underlying disease and a history of smoking and drinking; these patients ultimately died of the infection. These findings suggest that age may not be related to prognosis; however, underlying disease does appear to impact prognosis.
The patients who recovered had no dyspnea, whereas those who died developed different degrees of dyspnea. In terms of laboratory testing, compared to the patients who died, the patients who recovered had only slightly varying platelet counts and CK and PO<SUB>2</SUB> levels. In terms of complications, 1 patient who recovered had no complications, whereas the other developed secondary bacterial infections. In contrast, patients who died had 2?4 complications each. The time to treatment with oseltamivir therapy in the recovered cases was days 7 and 8, whereas it was days 3?8 in the patients who died. The above evidence implies that smoking, drinking, and underlying diseases may be related to the prognosis of H7N9 infection; dyspnea, decreased platelet counts, elevated CK levels, and hypoxemia may be associated with poor prognosis. The time to initiation of antiviral therapy seems to have no impact on prognosis, whereas the presence of complications may be associated with poor prognosis [10].
In conclusion, the first ever patient infected with H7N9 was treated at the Fifth People?s Hospital of Shanghai in February 2013. Within a 2-week period, several other cases of H7N9 infection emerged around 2 markets near the hospital. Fever, cough, sputum with blood, low lymphocyte counts, elevated CK and LDH levels, and pulmonary exudative lesions are significant characteristics of H7N9 infection, which easily progresses to ARDS. Among the cases, there was family clustering, which led to a high suspicion of contagious respiratory virus infection. In the early stage, human infection with H7N9 can be diagnosed by RT-PCR and viral isolation from respiratory specimens. Smoking, drinking, underlying diseases, dyspnea, low platelet counts, elevated CK levels, hypoxemia, and complications may be related to poor prognosis. However, diagnosis and treatment may be delayed because of the limited experience with this infection and small number of cases, which were among the first cases of H7N9 infection to be identified. Future studies are needed to elucidate the pathogenicity, transmissibility, and clinical features of H7N9 infection. In addition, techniques for early diagnosis to enable early administration of antiviral therapy and determination of the the factors affecting prognosis require further investigation.
Acknowledgments
The authors would like to thank the Fifth People?s Hospital of Shanghai and Shanghai Public Health Clinical Center, Fudan University for providing medical resources.
Author Contributions
Conceived and designed the experiments: ZJ YG. Performed the experiments: JS JX ZH YWH YCH QH BL WH YS FL. Analyzed the data: JS YCH ZJ. Contributed reagents/materials/analysis tools: JS JX ZH YWH YCH QH BL WH YS FL YLS CB. Wrote the manuscript: JS ZJ.
References
- 1.Webster RG, Geraci J, Petursson G, Skirnisson K (1981) Conjunctivitis in human beings caused by influenza A virus of seals. N Engl J Med 304: 911. doi:10.1056/NEJM198104093041516. PubMed: 7207529.
- 2.Eames KT, Webb C, Thomas K, Smith J, Salmon R et al. (2010) Assessing the role of contact tracing in a suspected H7N2 influenza A outbreak in humans in Wales. BMC Infect Dis 28; 10: 141. doi:10.1186/1471-2334-10-141.
- 3.Nguyen-Van-Tam JS, Nair P, Acheson P, Baker A, Barker M et al. (2006) Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill 11: E060504.2. PubMed: 16816456.
- 4.Puzelli S, Di Trani L, Fabiani C, Campitelli L, De Marco MA et al. (2003) Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J Infect Dis 192: 1318-1322. PubMed: 16170747.
- 5.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H et al. (2004) Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363: 587-593. doi:10.1016/S0140-6736(04)15589-X. PubMed: 14987882.
- 6.Tweed SA, Skowronski DM, David ST, Larder A, Petric M et al. (2004) Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis 10: 2196-2199. doi:10.3201/eid1012.040961. PubMed: 15663860.
- 7.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA et al. (2004) Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101: 1356-1361. doi:10.1073/pnas.0308352100. PubMed: 14745020.
- 8.Yu H, Cowling BJ, Feng L, Lau EH, Liao Q et al. (2013) Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 382(9887): 138-145. doi:10.1016/S0140-6736(13)61207-6. PubMed: 23803487.
- 9.Gao R, Cao B, Hu Y, Feng Z, Wang D et al. (2013) Human Infection with a Novel Avian-Origin Influenza A (H7N9) Virus. N Engl J Med 368: 1888-1897. doi:10.1056/NEJMoa1304459. PubMed: 23577628.
- 10.Wu C, Lu X, Wang X, Jin T, Cheng X et al. (2013) Clinical symptoms, immune factors, and molecular characteristics of an adult male in Shenzhen, China infected with influenza virus H5N1. J Med Virol 85: 760-768. doi:10.1002/jmv.23492. PubMed:23508902.
- 11.Gao HN, Lu HZ, Cao B, Du B, Shang H et al. ( May 222013) Clinical Findings in 111 Cases of Influenza A (H7N9) Virus Infection. N Engl J Med May 22. PubMed:23697469.
- 12.Belser JA, Davis CT, Balish A, Edwards LE, Zeng H et al. (2013) Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J Virol 87: 5746-5754. doi:10.1128/JVI.00154-13. PubMed: 23487452.
- 13.Gonz?lez-Reiche AS, Morales-Betoulle ME, Alvarez D, Betoulle JL, M?ller ML et al. (2012) Influenza a viruses from wild birds in Guatemala belong to the North American lineage. PLOS ONE 7: e32873. doi:10.1371/journal.pone.0032873. PubMed: 22427902.
- 14.Bertran K, P?rez-Ram?rez E, Busquets N, Dolz R, Ramis A et al. (2011) Pathogenesis and transmissibility of highly (H7N1) and low (H7N9) pathogenic avian influenza virus infection in red-legged partridge (Alectoris rufa). Vet Res 7: 42 (1): 24 doi:10.1186/1297-9716-42-24.
- 15.Joseph T, McAuliffe J, Lu B, Jin H, Kemble G et al. (2007) Evaluation of replication and pathogenicity of avian influenza a H7 subtype viruses in a mouse model. J Virol 81: 10558-10566. doi:10.1128/JVI.00970-07. PubMed: 17634234.
- 16.Katz JM, Lim W, Bridges CB, Rowe T, Hu-Primmer J et al. (1999) Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis 180: 1763-1770. doi:10.1086/315137. PubMed: 10558929.
- 17.Buxton Bridges C, Katz JM, Seto WH, Chan PK, Tsang D et al. (2000) Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis 181: 344-348. doi:10.1086/315213. PubMed: 10608786.
- 18.Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W et al. (2005) Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 352: 333-340. doi:10.1056/NEJMoa044021. PubMed: 15668219.
- 19.Ostrowsky B, Huang A, Terry W, Anton D, Brunagel B et al. (2012) Low pathogenic avian influenza A (H7N2) virus infection in immunocompromised adult, New York, USA, 2003. Emerg Infect Dis 18: 1128-1131. doi:10.3201/eid1807.111913. PubMed:22710273.
- 20.Kurtz J, Manvell RJ, Banks J (1996) Avian influenza virus isolated from a woman with conjunctivitis. Lancet 348: 901-902. doi:10.1016/S0140-6736(05)64783-6. PubMed:8826845.
- 21.Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF et al. (2004) Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363: 617-619. doi:10.1016/S0140-6736(04)15595-5. PubMed: 14987888.
- 22.Kemink SA, Fouchier RA, Rozendaal FW, Broekman JM, Koopmans M et al. (2004) A fatal infection due to avian influenza-A (H7N7) virus and adjustment of the preventive measures. Ned Tijdschr Geneeskd 148: 2190-2194. PubMed: 15559415.
- 23.Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL et al. (1998) Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351: 467-471. doi:10.1016/S0140-6736(98)01182-9. PubMed: 9482437.
- 24.Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM et al. (2004) Avian influenza A (H5N1) in 10 patients in Vietnam. N Eng l: 1179?88 J Med 350: 1179-1188 PubMed:14985470.
- 25.Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P et al. (2005) Human disease from influenza A (H5N1) , Thailand, 2004. Emerg Infect Dis 11: 201-209. doi:10.3201/eid1102.041061. PubMed: 15752436.
-
------
Comment