Weekly U.S. Influenza Surveillance Report

Note: CDC is tracking the COVID-19 pandemic in a weekly publication called COVID Data Tracker Weekly Review.
Key Updates for Week 52, ending January 1, 2022
Seasonal influenza activity in the United States is increasing, including indicators that track hospitalizations. The amount of activity varies by region.
Viruses
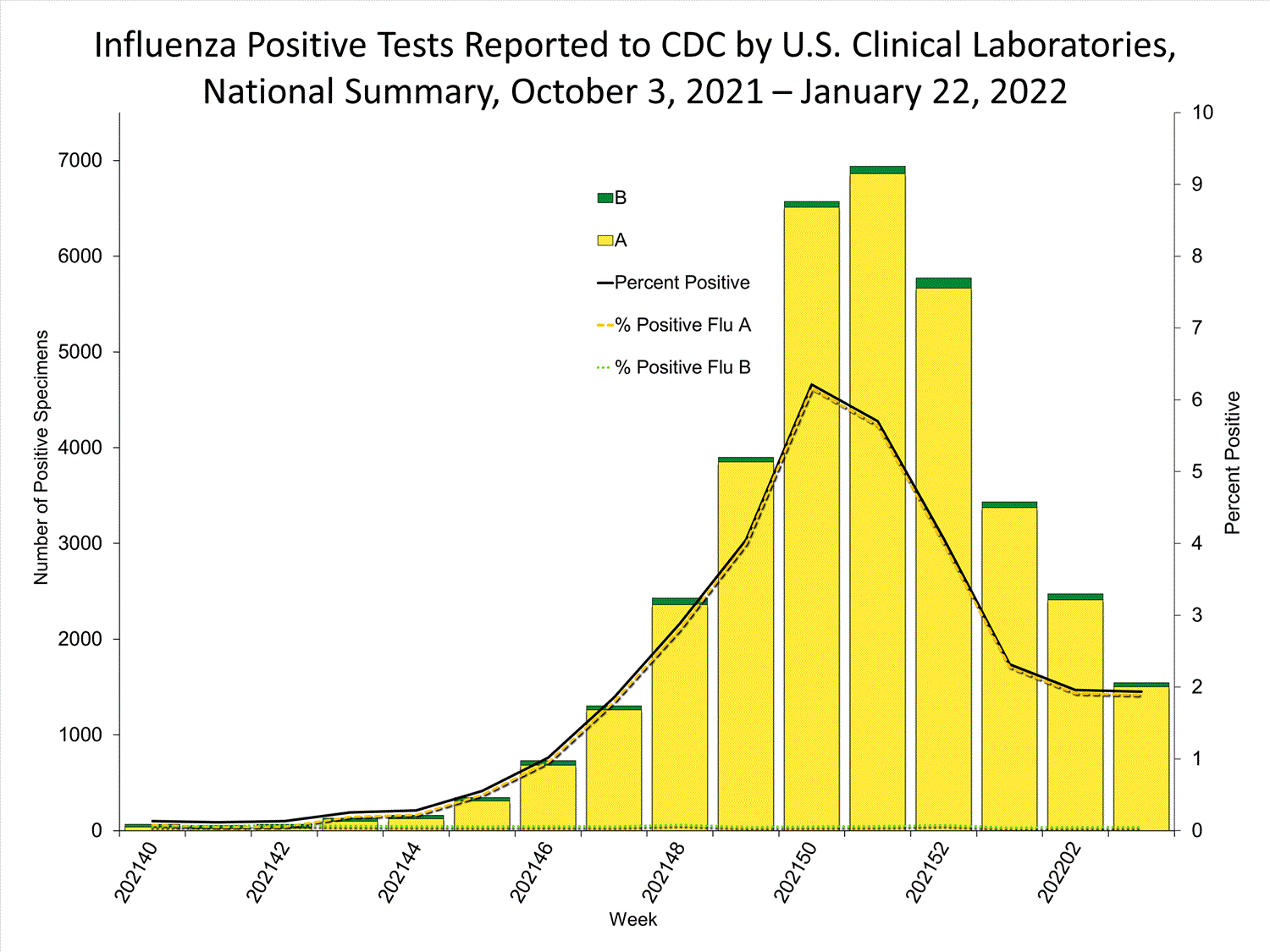
Clinical Lab3.8%
positive for influenza
this week
Public Health Lab
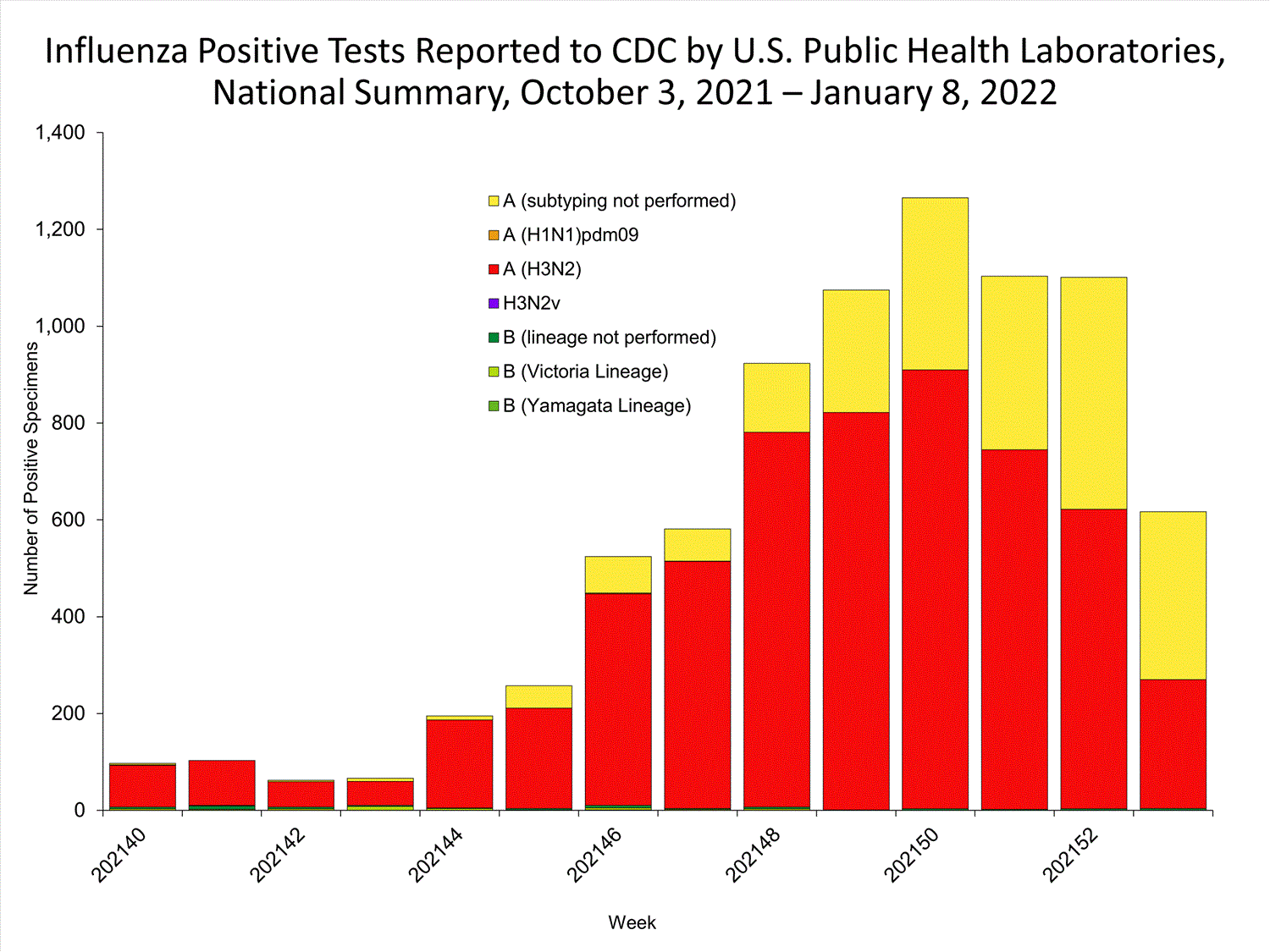
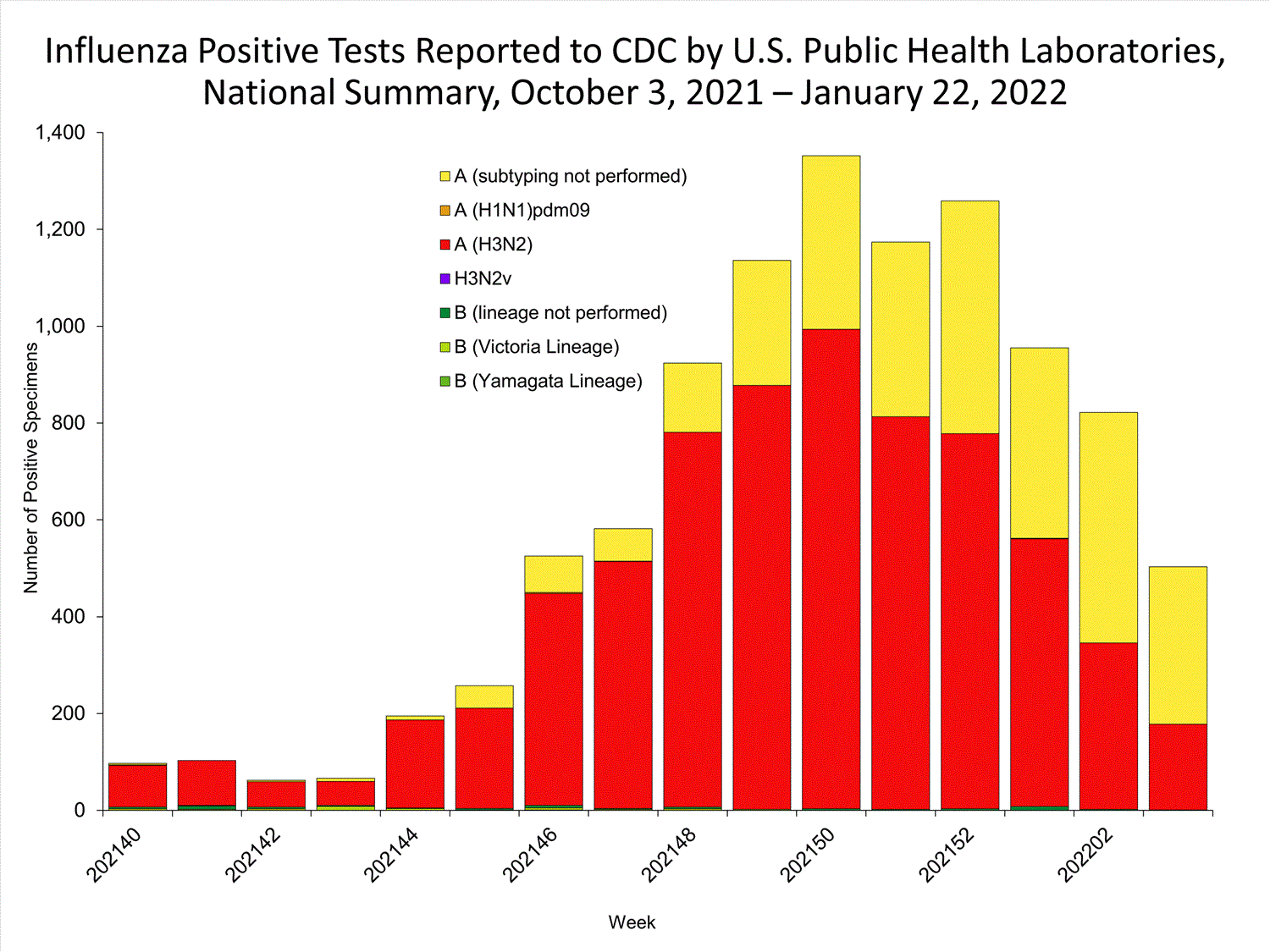
The majority of viruses
detected are influenza A(H3N2).
Virus Characterization
Genetic characterization data are now being reported.
Illness
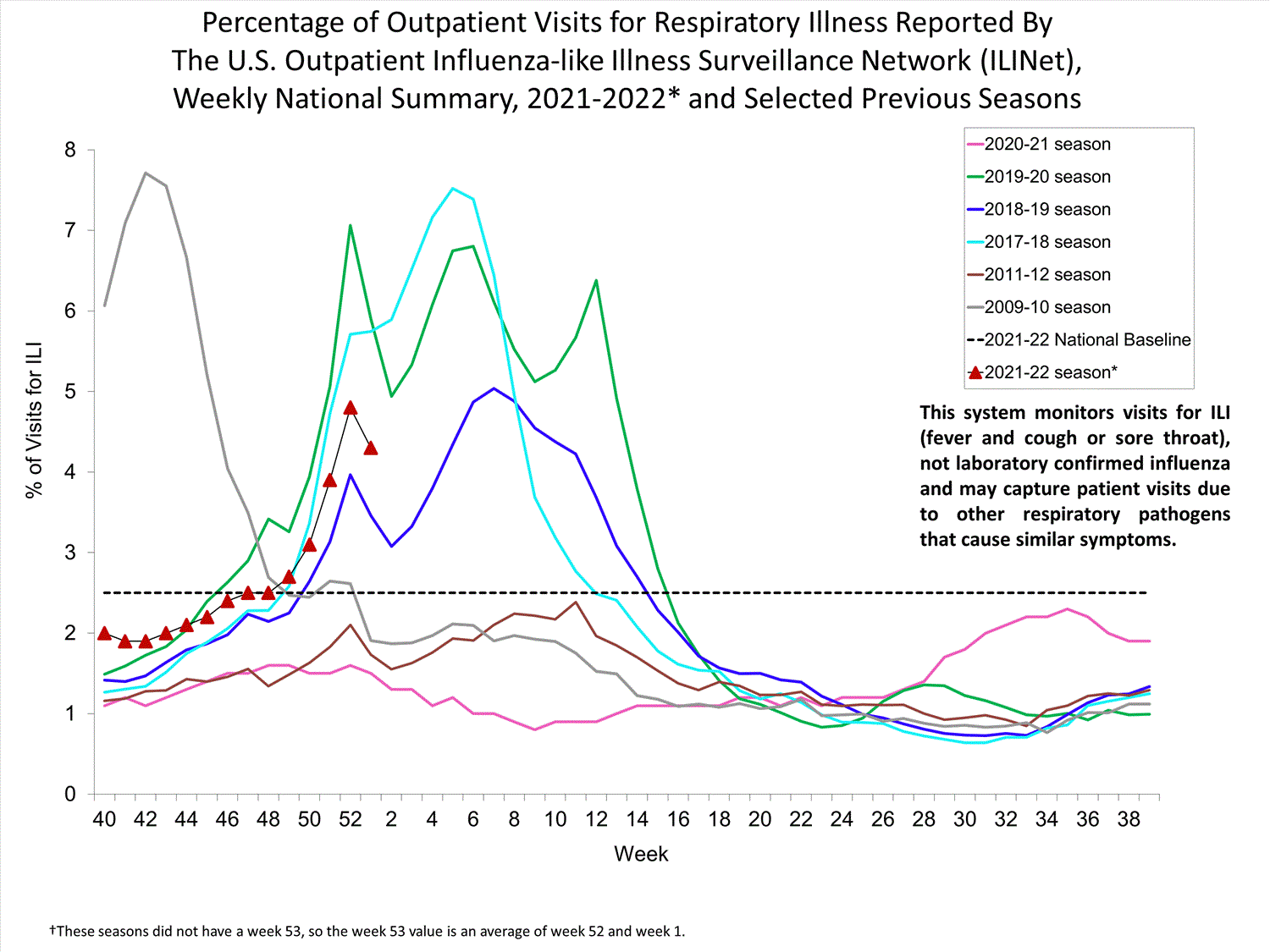
Outpatient Respiratory Illness4.8%
of visits to a health care provider are for respiratory illness this week
(above baseline)
Outpatient Respiratory Illness: Activity Map
This week, 10 jurisdictions experienced moderate activity and 31 jurisdictions experienced high or very high activity.
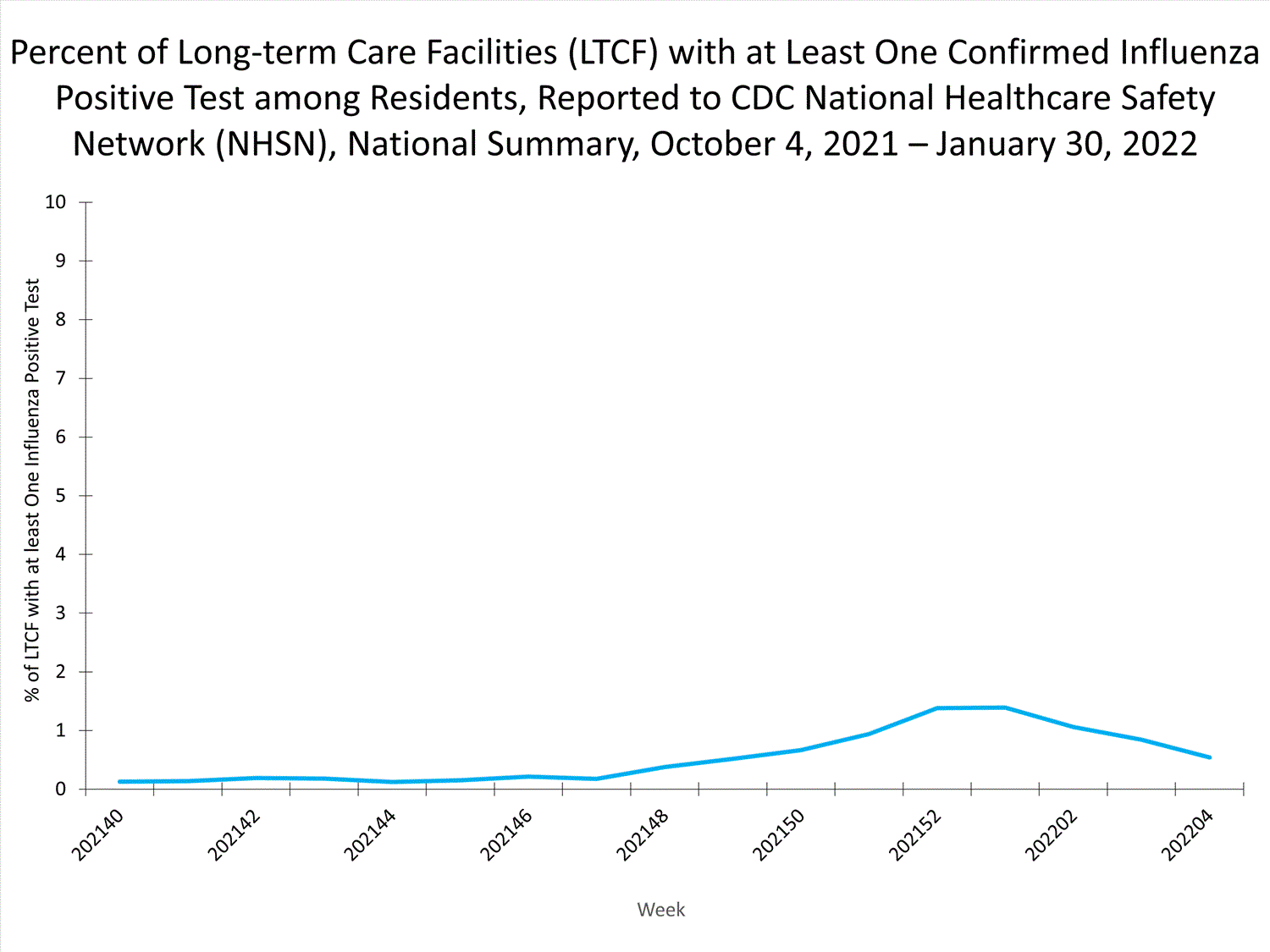
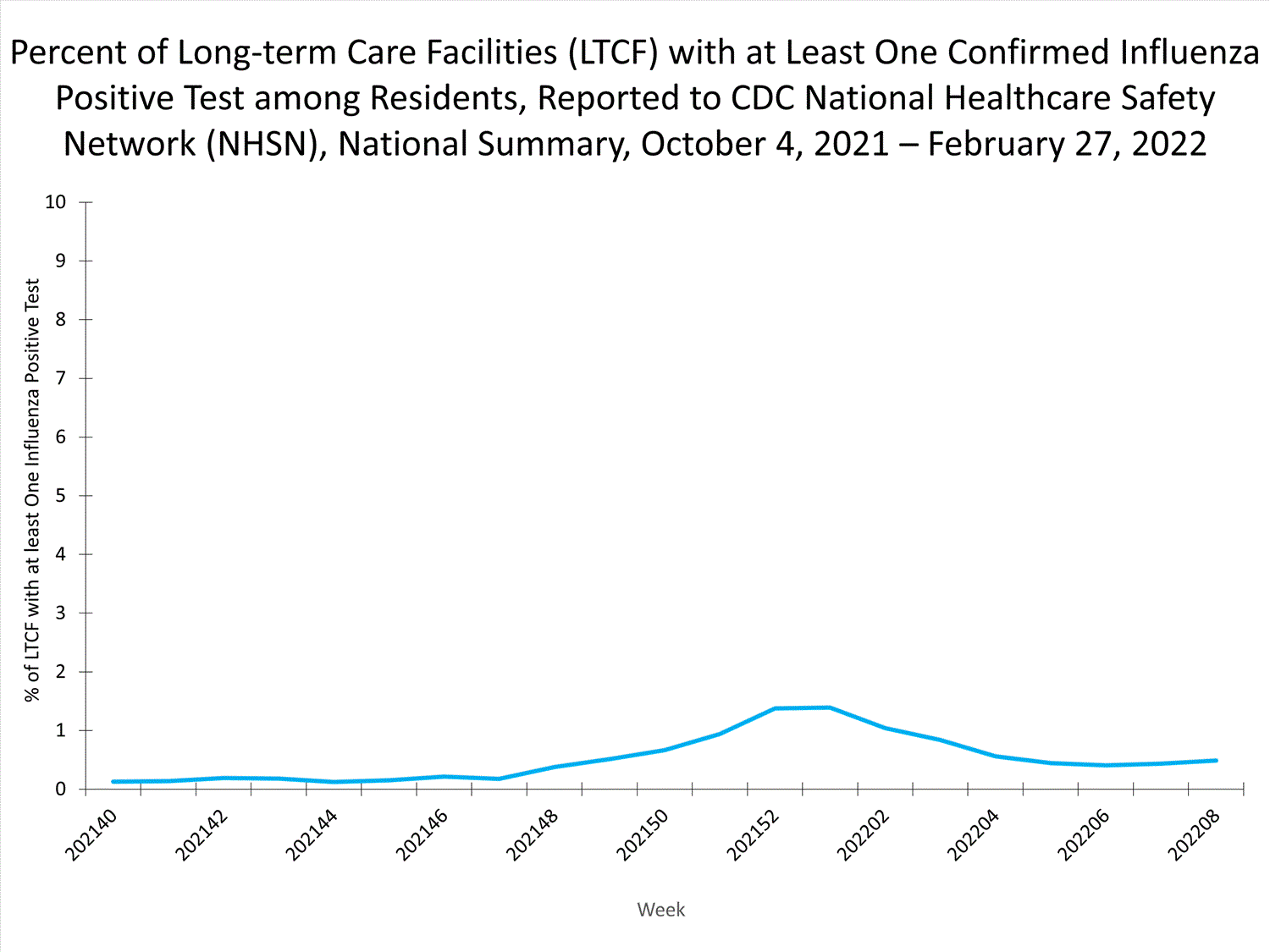
Long-term Care Facilities1.3%
of facilities reported
≥ 1 influenza-positive test
among residents this week.
Severe Disease
FluSurv-NET2.6 per 100,000
cumulative hospitalization rate
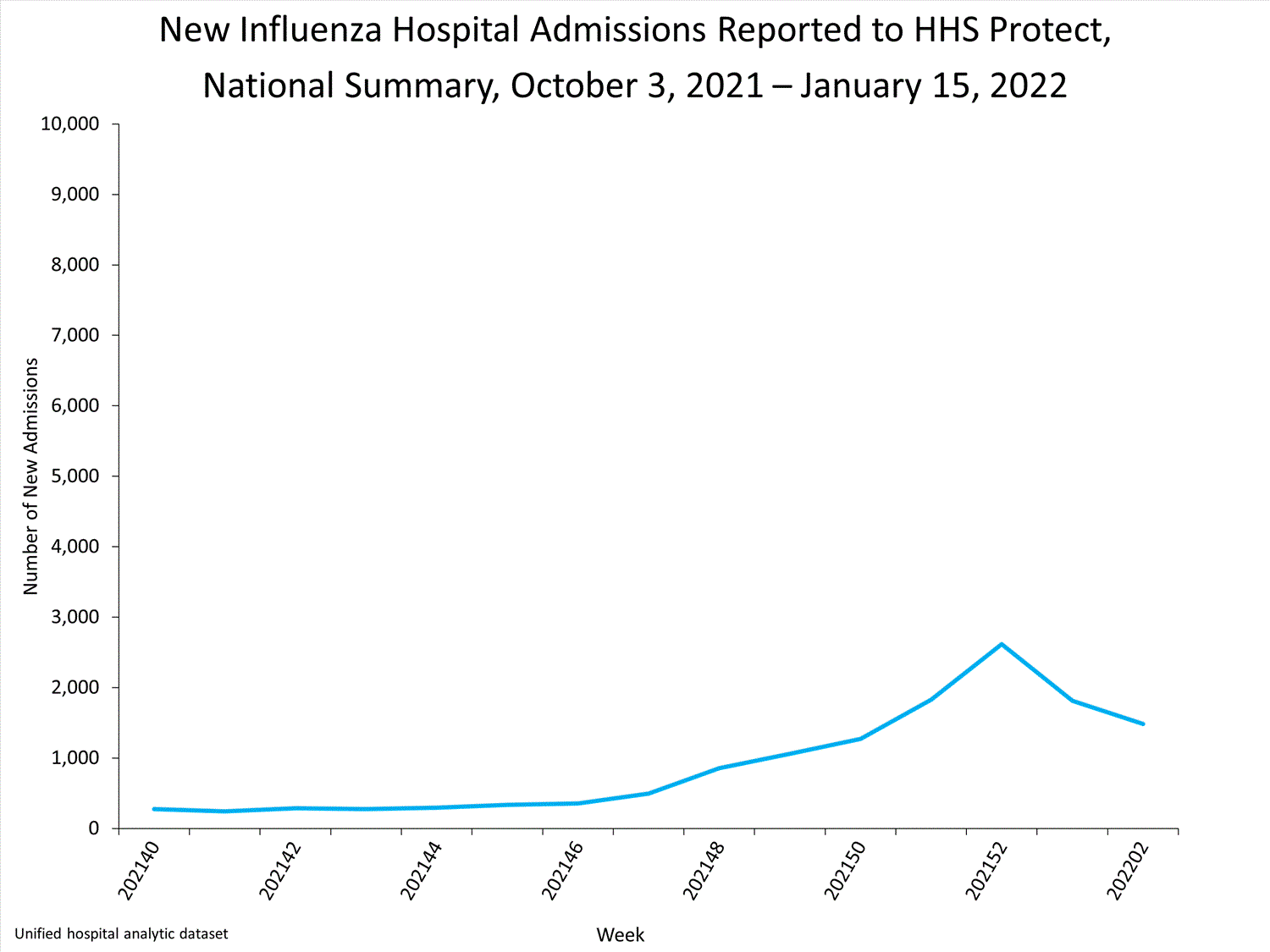
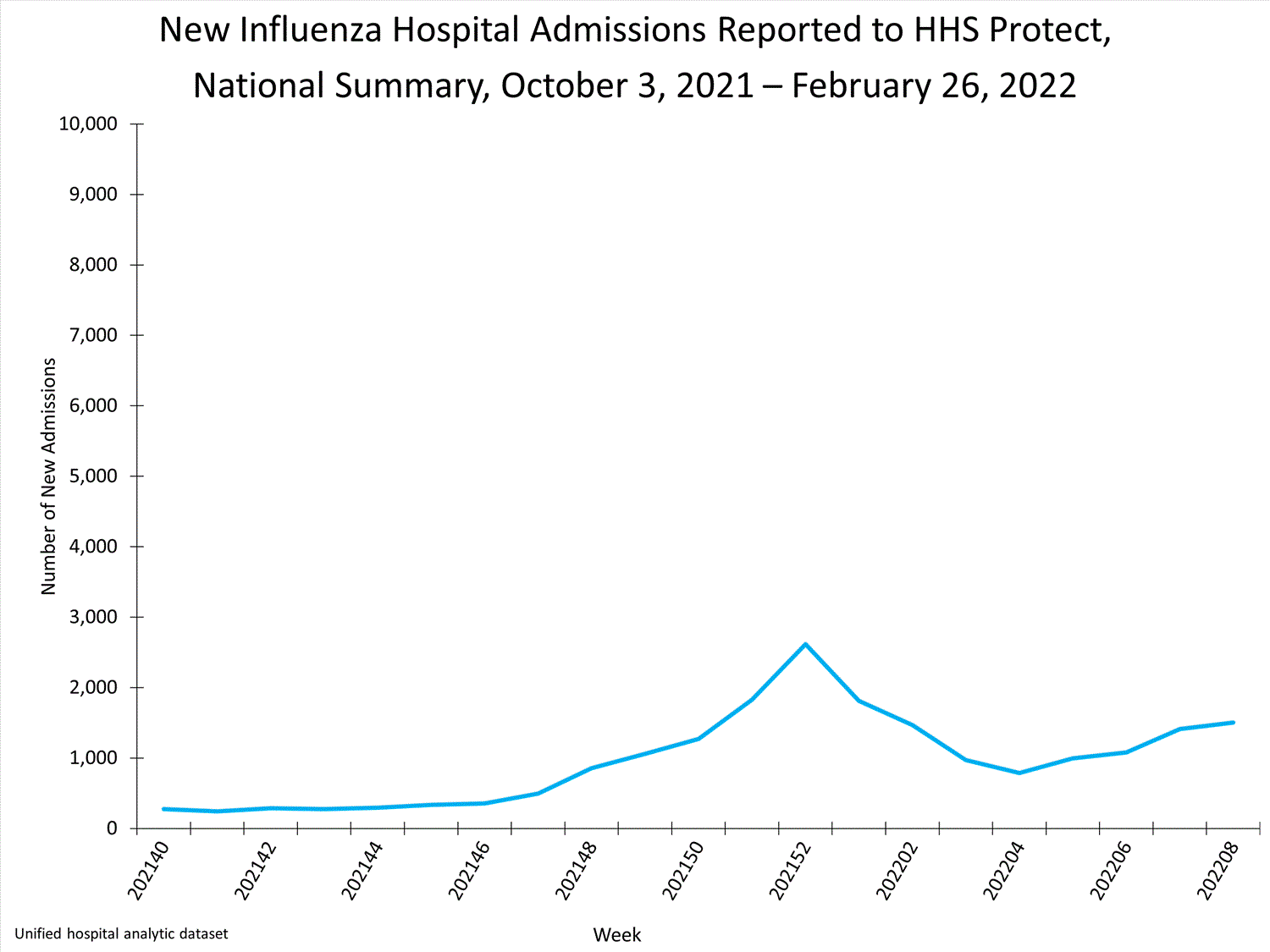
HHS Protect Hospitalizations2,615
patients admitted to hospitals with influenza
this week.
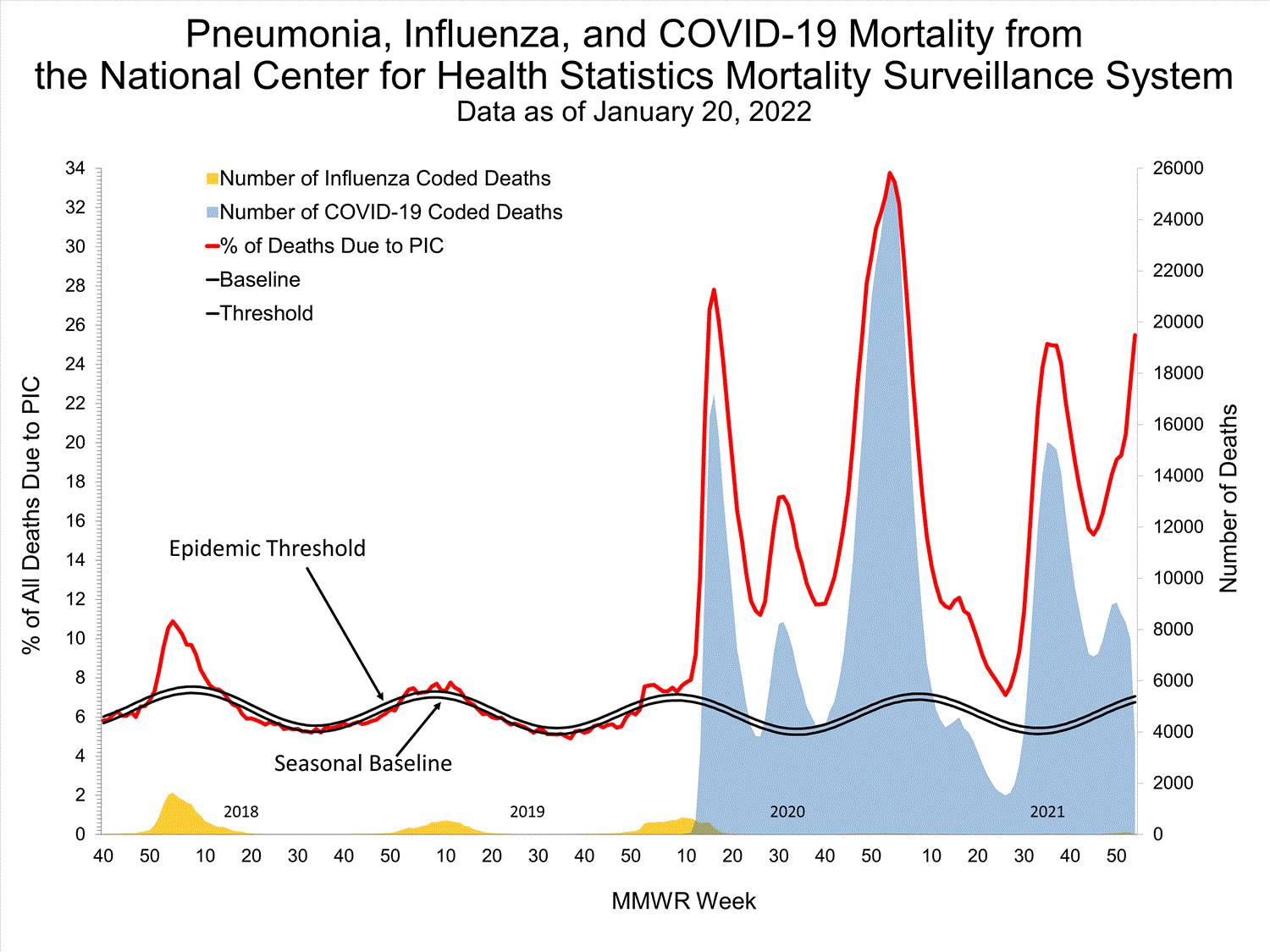
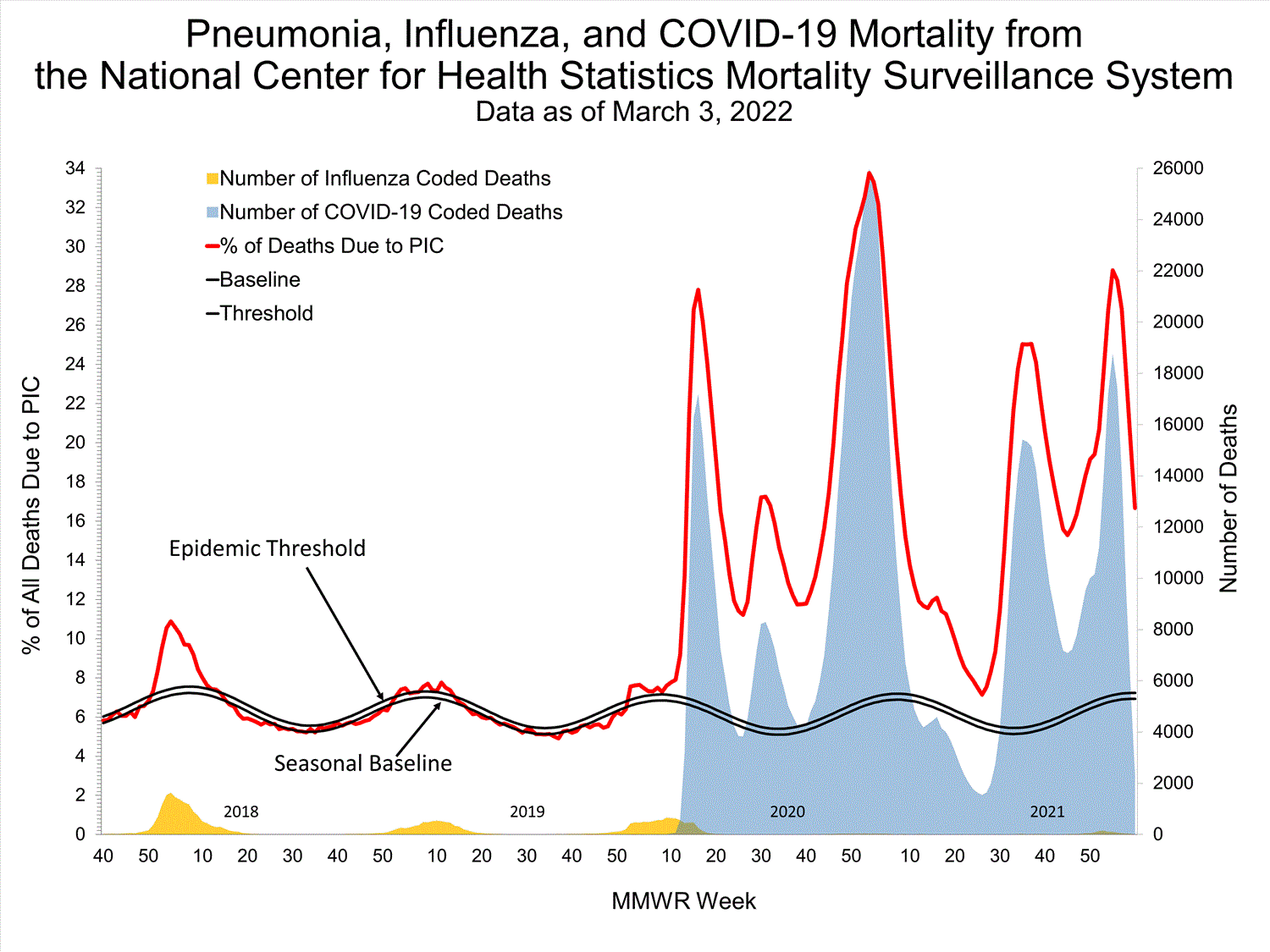
NCHS Mortality19.9%
of deaths attributed to pneumonia, influenza, or COVID-19 this week (above threshold)
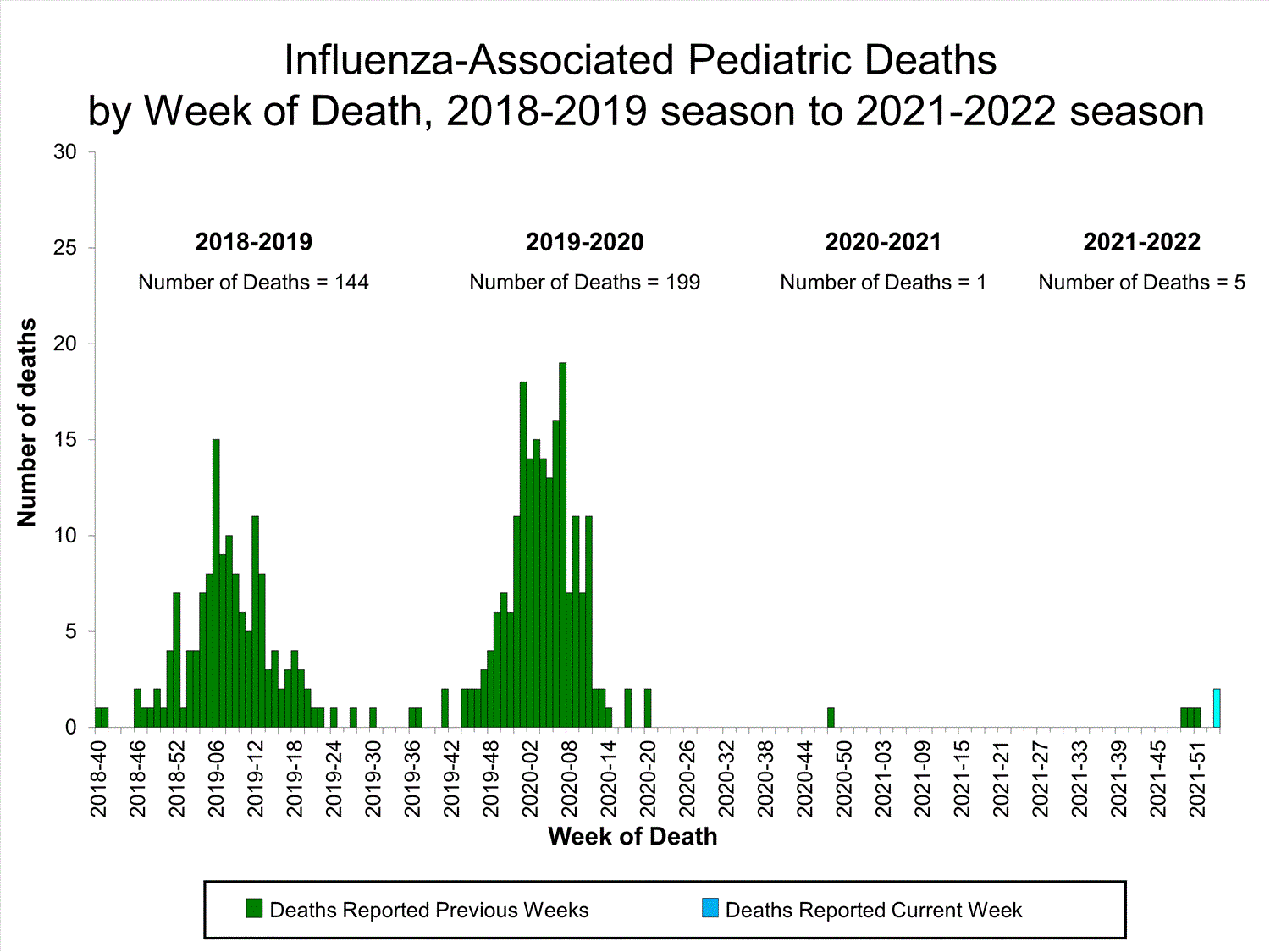
Pediatric Deaths0
influenza-associated deaths reported this week
All data are preliminary and may change as more reports are received.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component is available on the surveillance methods page.
Additional information on the current and previous influenza seasons for each surveillance component are available on FluView Interactive.
Key Points
- Influenza activity is increasing, with the eastern and central parts of the country seeing the majority of viruses reported and the western part of the country reporting lower levels of influenza virus circulation.
- The majority of influenza viruses detected are A(H3N2). Earlier in the season, most influenza A(H3N2) infections occurred among children and young adults ages 5-24 years; however, in recent weeks, the proportion of infections occurring among other age groups, especially adults age 25 years and older, has been increasing.
- Most of the H3N2 viruses so far are genetically closely related to the vaccine virus, but there are some antigenic differences that have developed as H3N2 viruses have continued to evolve.
- The percentage of outpatient visits due to respiratory illness continues to increase and is above the national baseline. Influenza is contributing to levels of respiratory illness, but other respiratory viruses are also circulating. The relative contribution of influenza varies by location.
- Hospitalizations for influenza continue to increase. The cumulative hospitalization rate in the FluSurv-NET system is higher than the rate for the entire 2020-2021 season, but lower than the rate seen at this time during the four seasons preceding the COVID-19 pandemic.
- The flu season is just getting started. There’s still time to get vaccinated. An annual flu vaccine is the best way to protect against flu and its potentially serious complications. CDC recommends everyone 6 months and older get a flu vaccine.
- There are early signs that flu vaccination uptake is down this season compared to last.
- Flu vaccines are available at many different locations, including pharmacies and health departments. With flu activity just getting started, there is still time to benefit from flu vaccination this season. Visit www.vaccines.gov to find a flu vaccine near you.
- There are also flu antiviral drugs that can be used to treat flu illness.
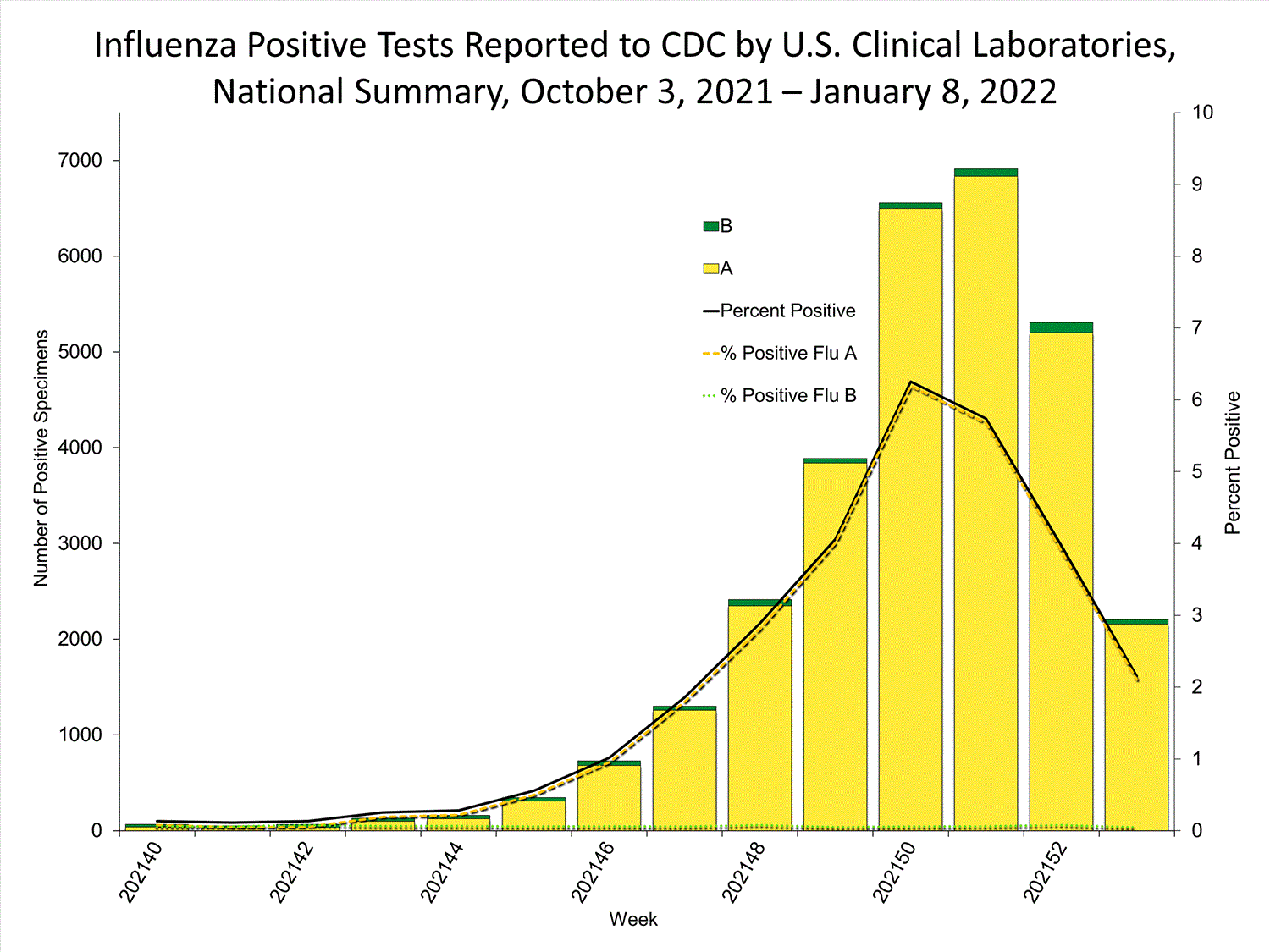
Reporting delays due to the holiday may have impacted week 52 virologic data; therefore, testing numbers and percent positivity should be interpreted with caution. As additional data are received, we expect to see an increase in the number of positive influenza tests, but we may not see a corresponding increase in percent positivity. While the number of influenza virus infections may be increasing, the number of respiratory illnesses due to other viruses such as SARS-CoV-2 is increasing more rapidly, resulting in the proportion of respiratory illness due to influenza, or percent positivity, to decrease.
Influenza A(H3N2) viruses have been the most frequently detected. Persons aged 5-24 years old account for the largest proportion of influenza A(H3N2) viruses detected, but the proportion of influenza A(H3N2) virus detections occurring among other age groups has increased in recent weeks. For regional and state level data about circulating influenza viruses, please visit FluView Interactive. Viruses known to be associated with recent live attenuated influenza vaccine (LAIV) receipt or found upon further testing to be a vaccine virus are not included as they are not circulating influenza viruses.
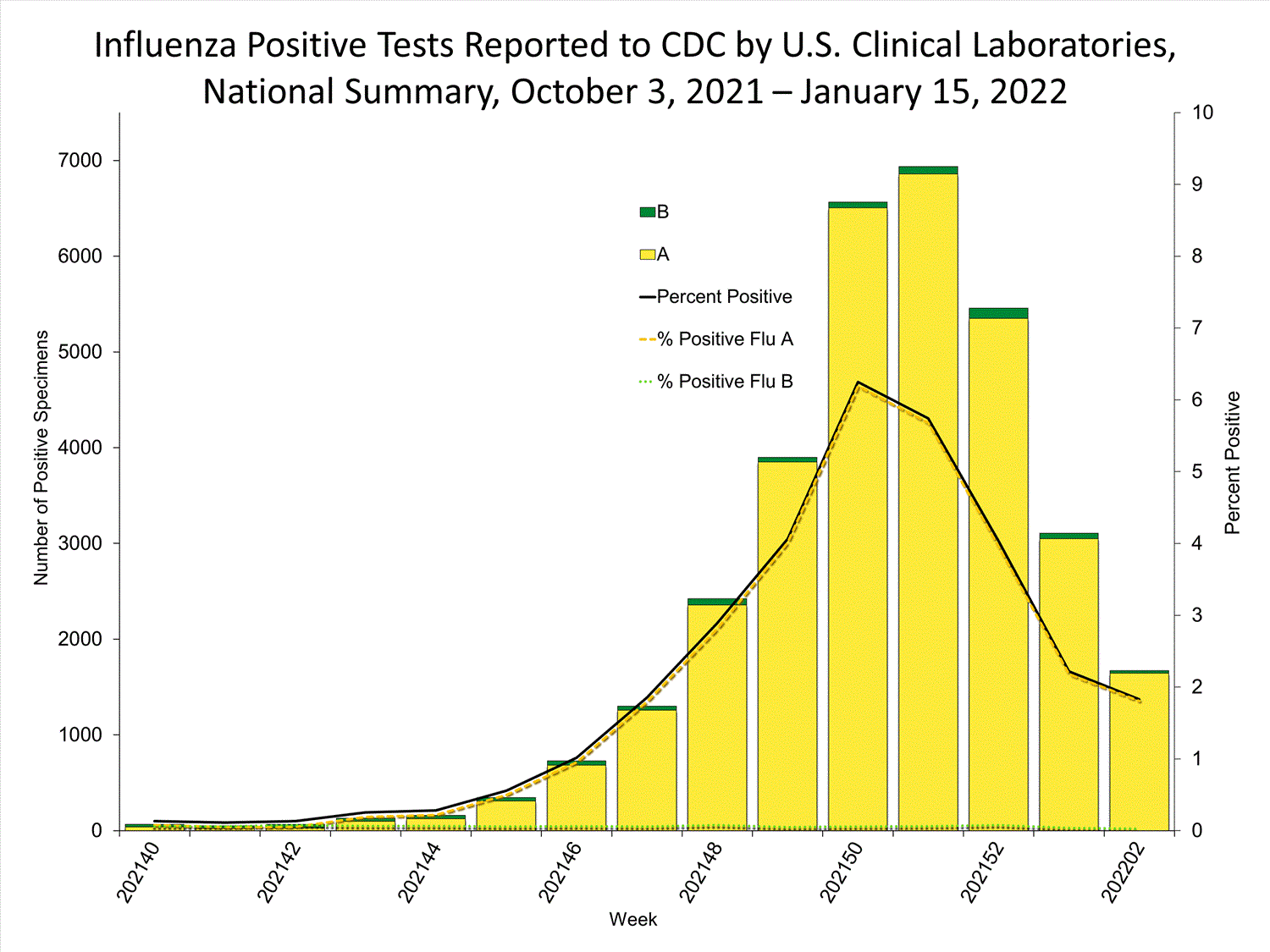
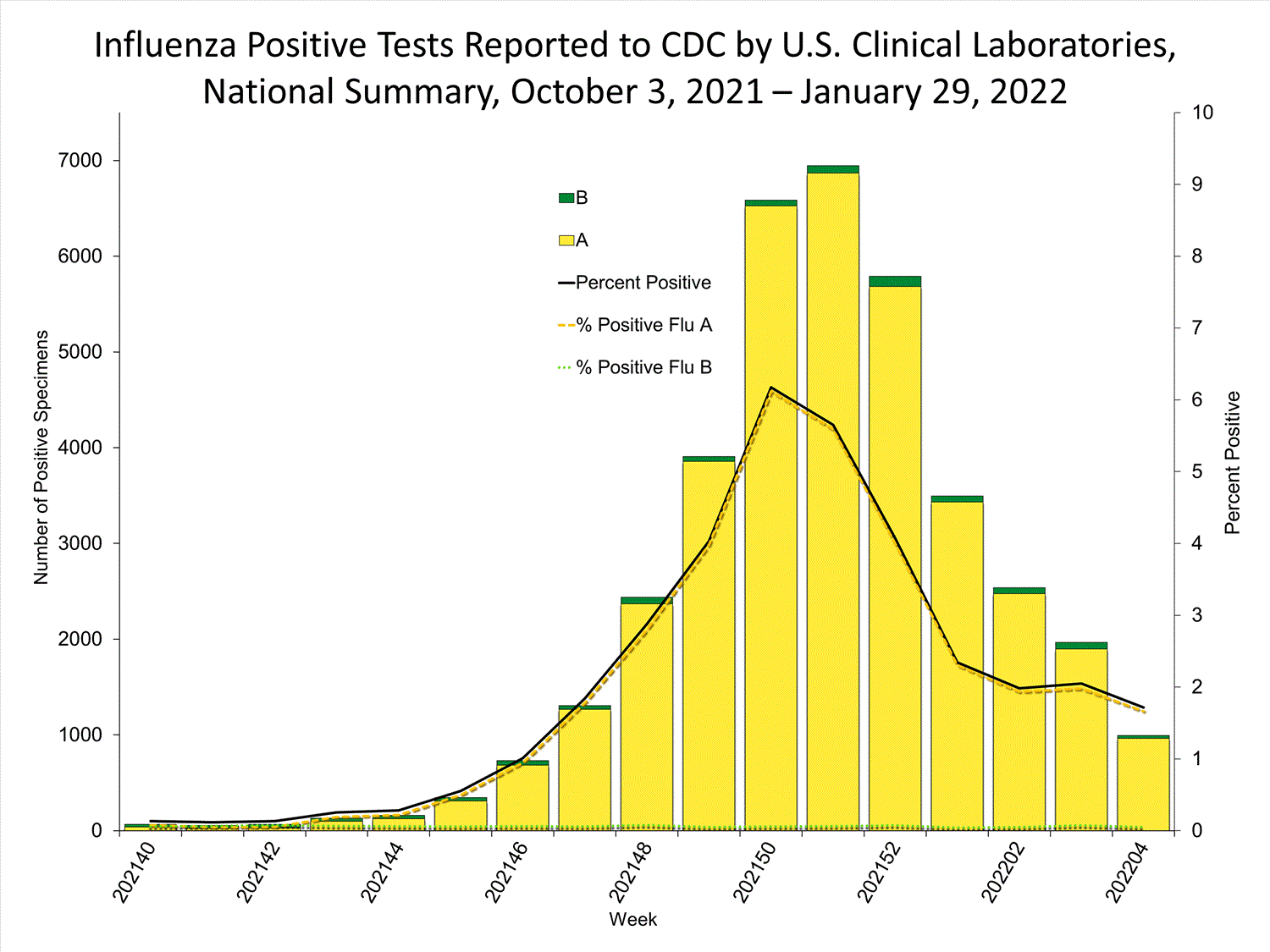
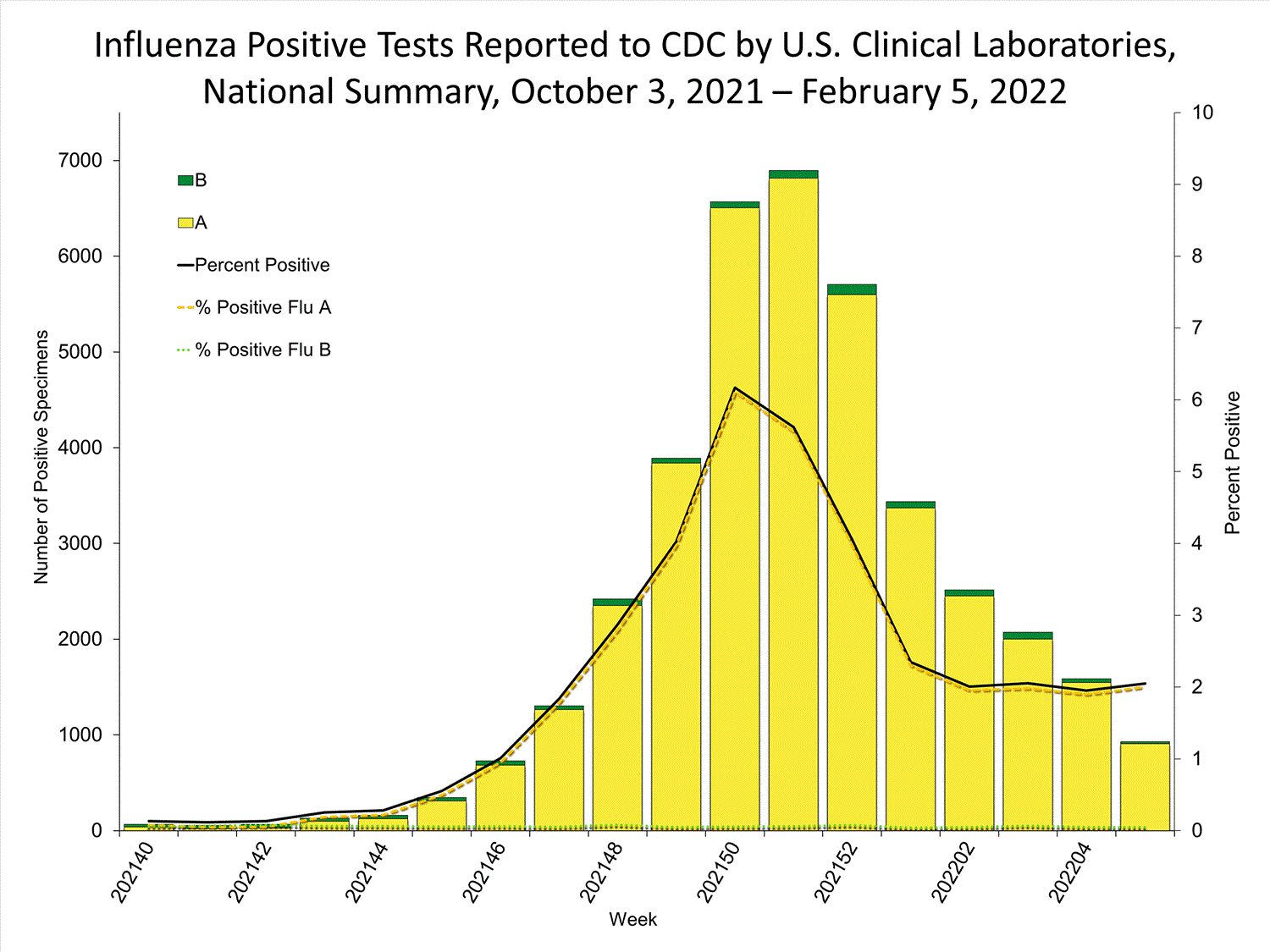
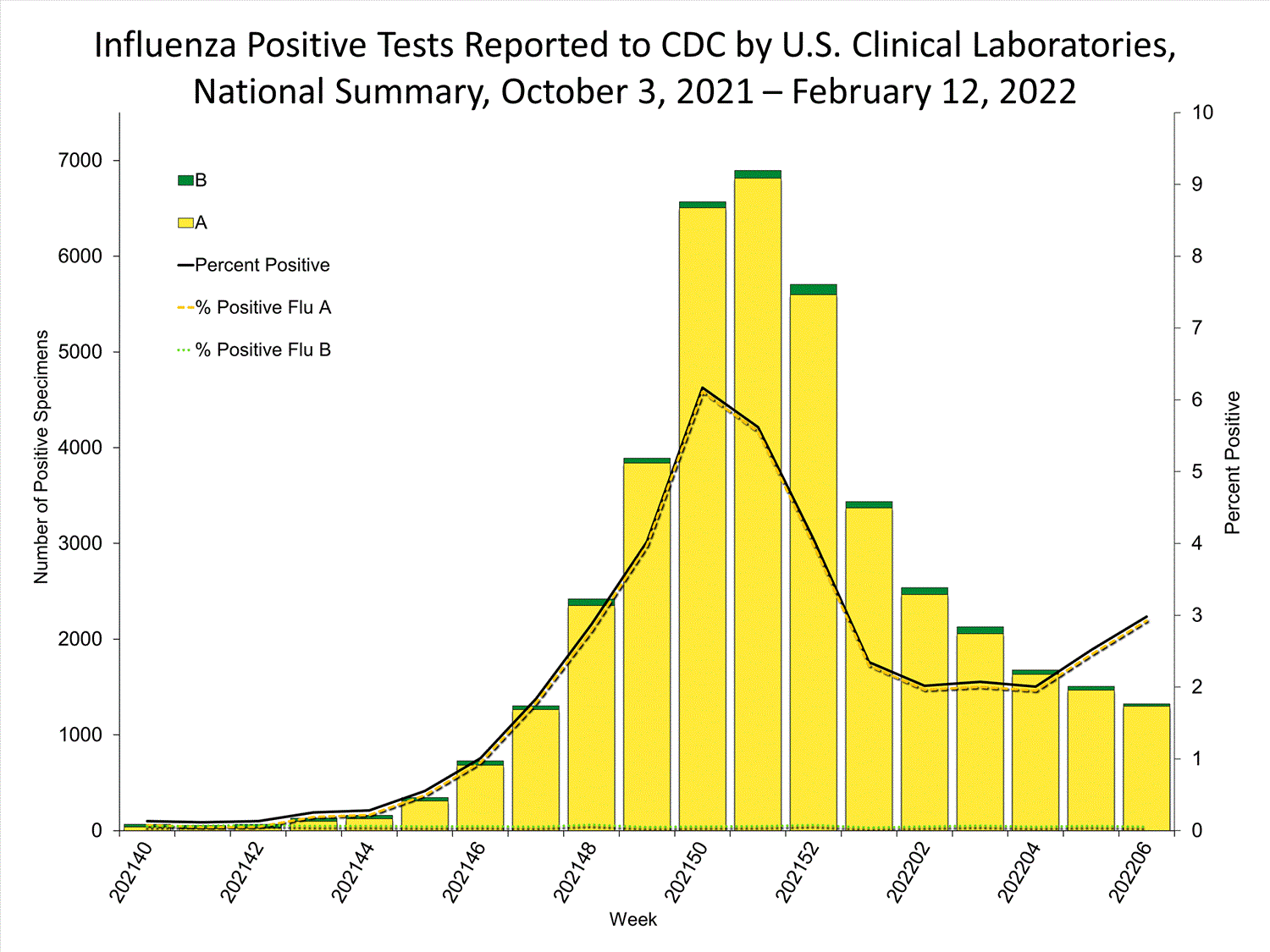
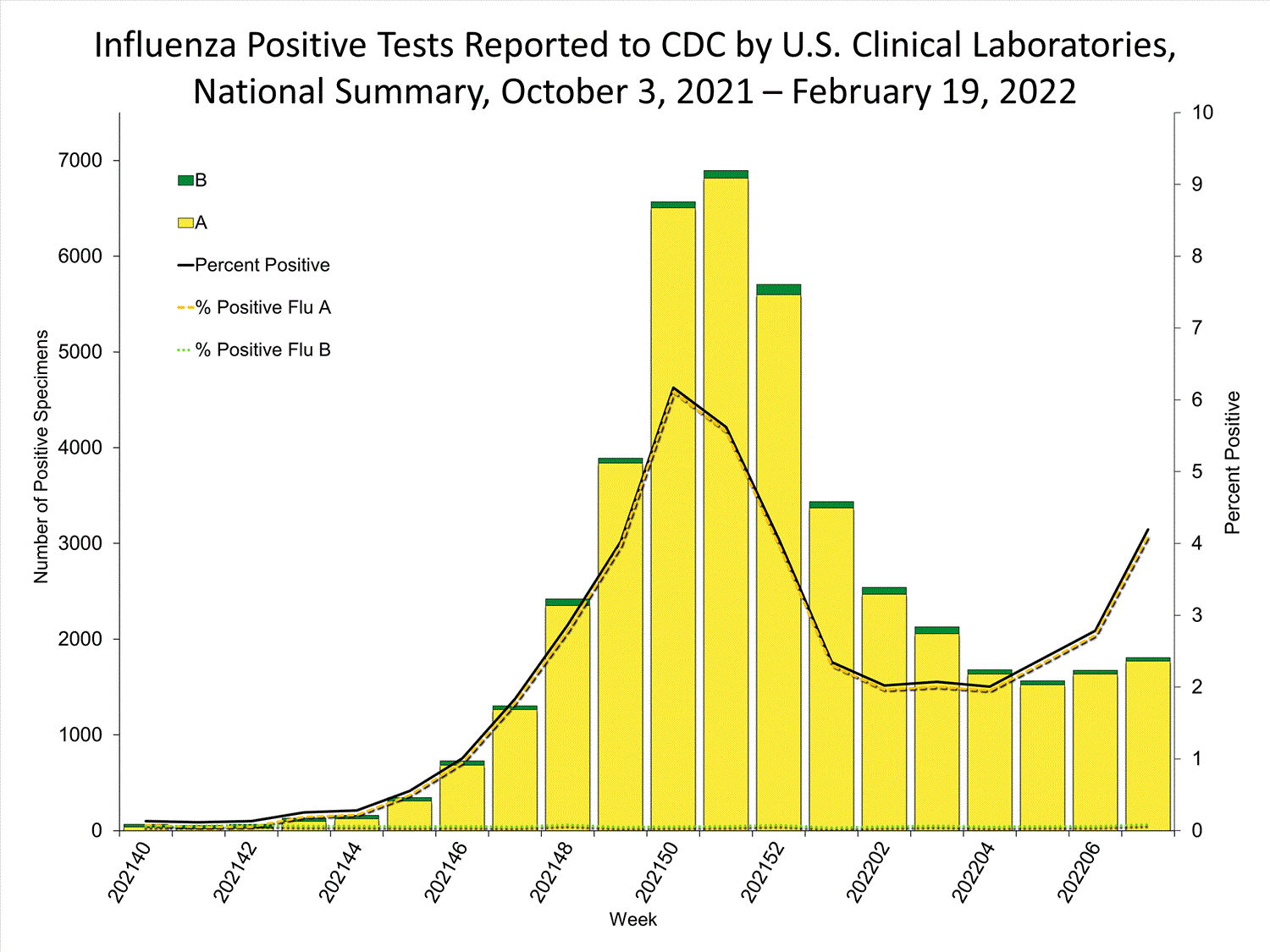
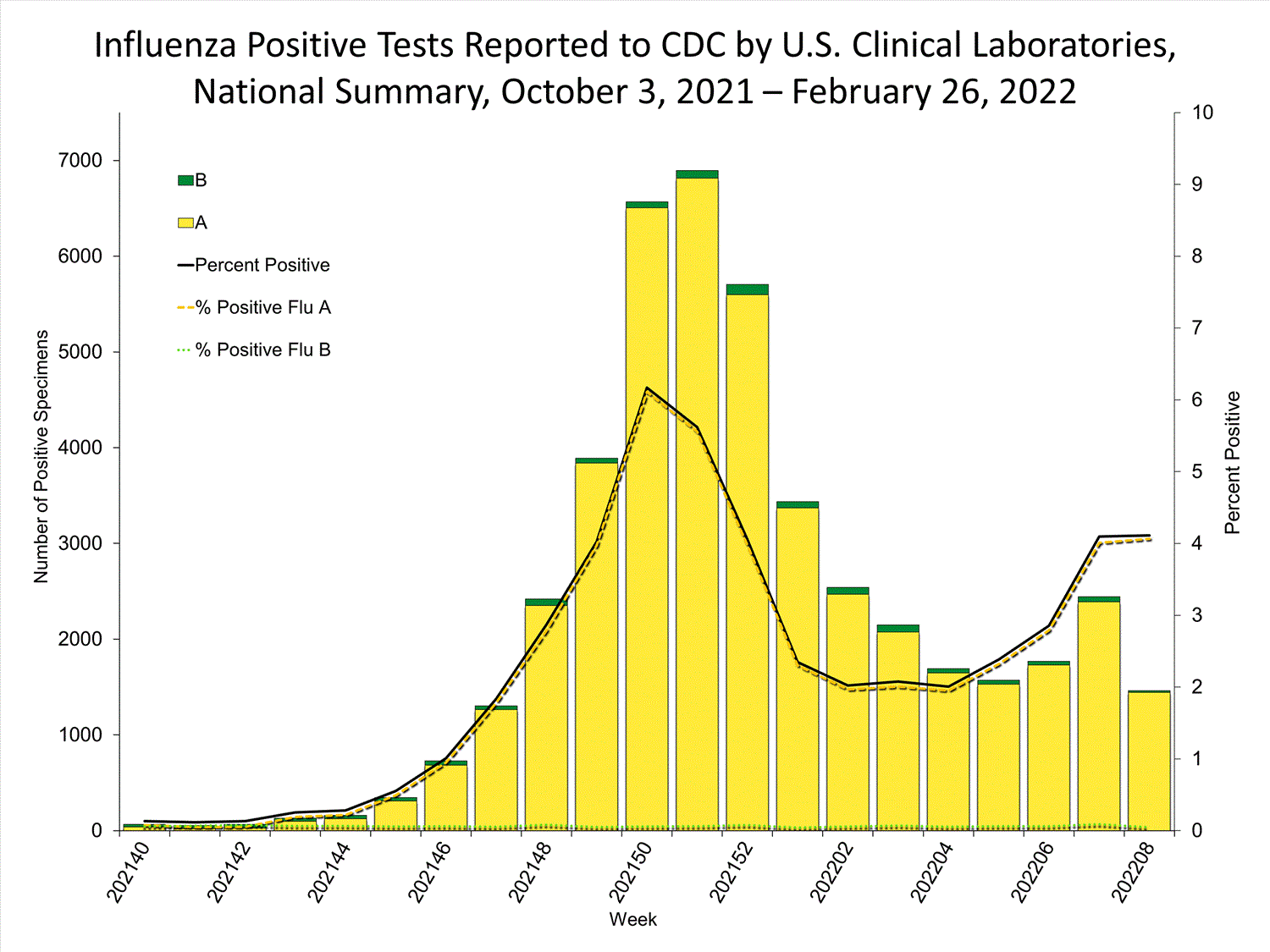
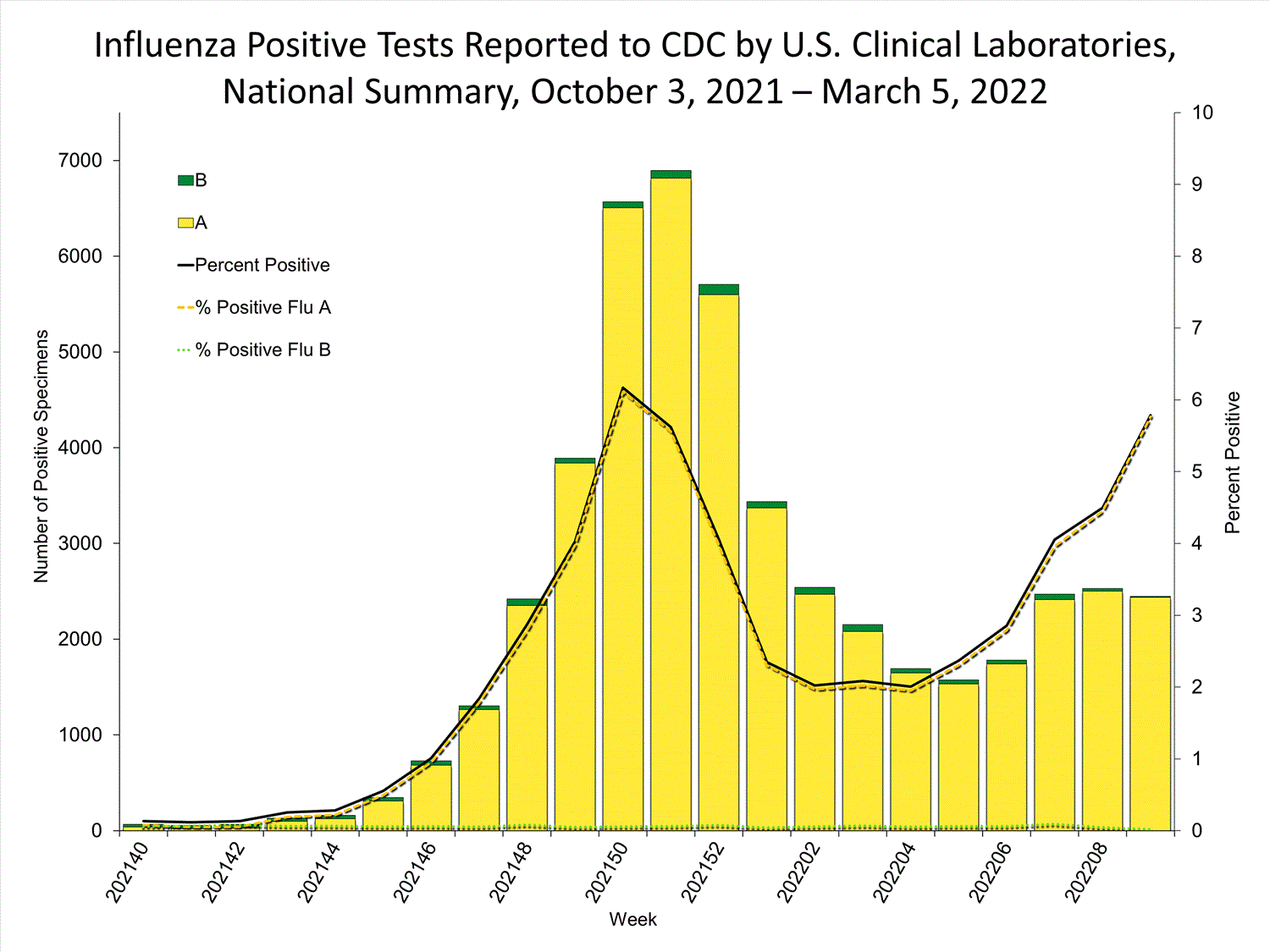
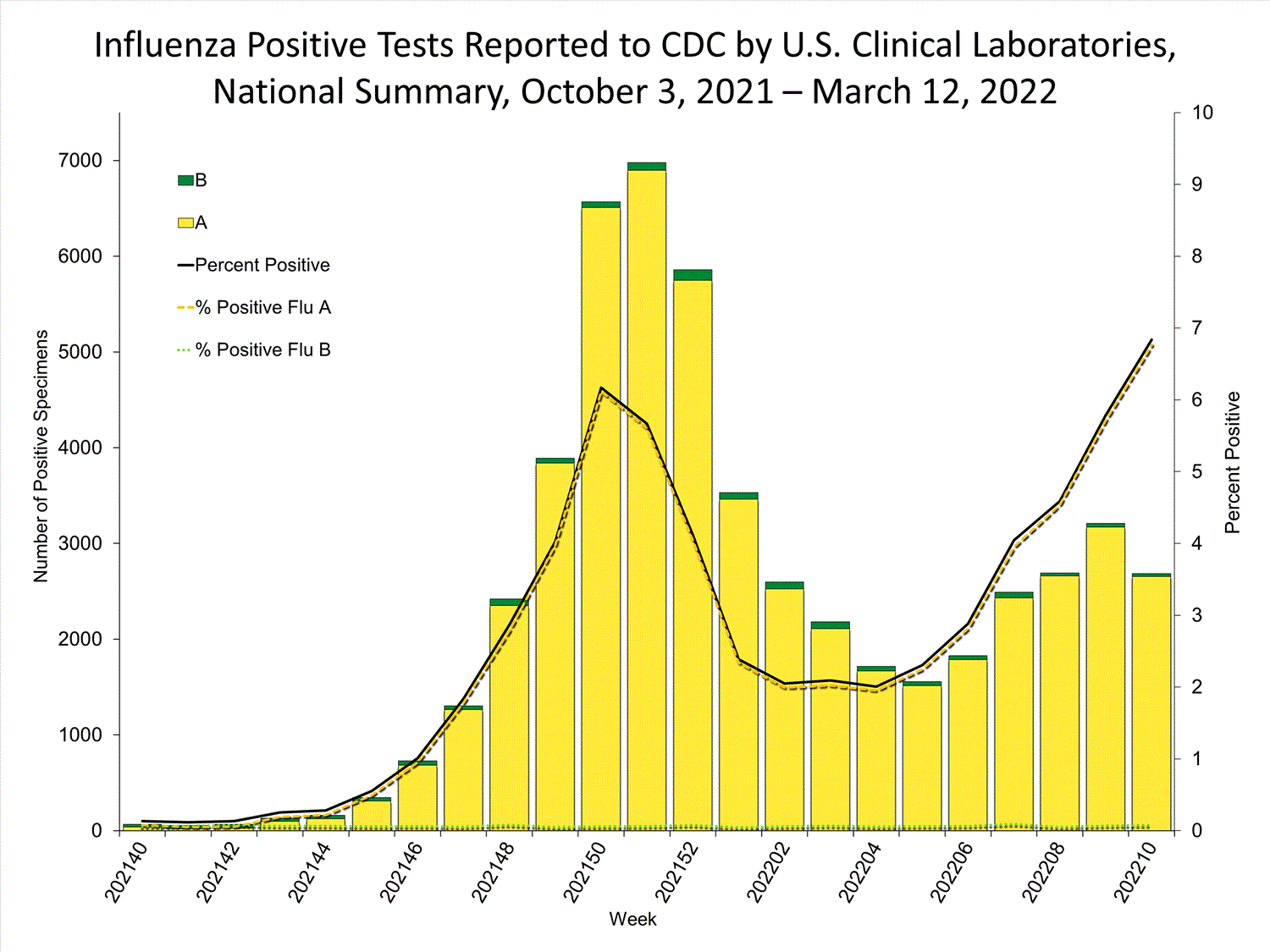
Clinical Laboratories
The results of tests performed by clinical laboratories nationwide are summarized below. Data from clinical laboratories (the percentage of specimens tested that are positive for influenza) are used to monitor whether influenza activity is increasing or decreasing.
| No. of specimens tested | 115,580 | 974,946 |
| No. of positive specimens (%) | 4,413 (3.8%) | 26,946 (2.8%) |
| Positive specimens by type | ||
| Influenza A | 4,329 (98.1%) | 26,328 (97.7%) |
| Influenza B | 84 (1.9%) | 618 (2.3%) |
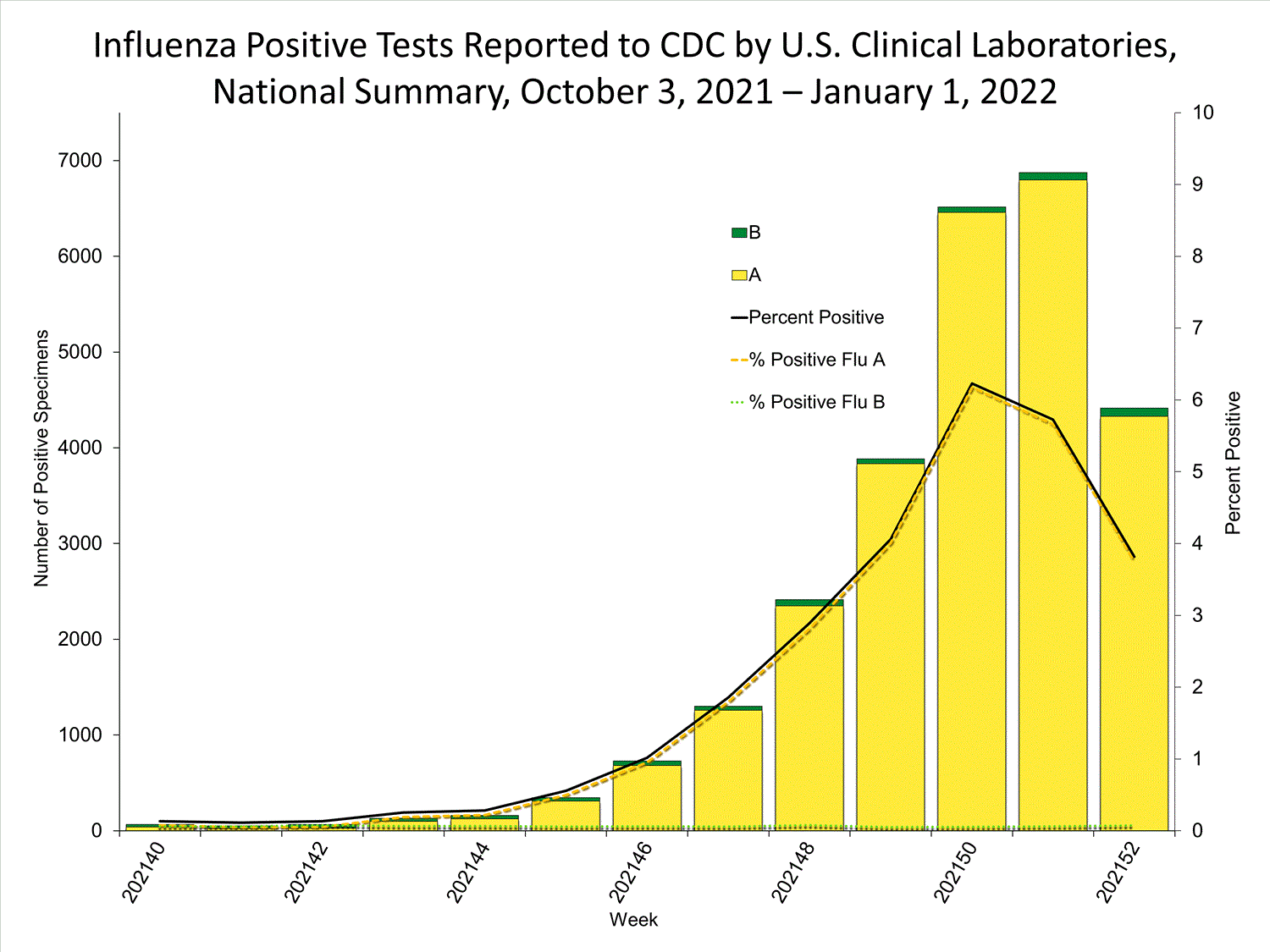 * Reporting delays due to the holiday may have impacted week 52 virologic data; therefore, testing numbers and percent positivity should be interpreted with caution. As additional data are received, we expect to see an increase in the number of positive influenza tests, but we may not see a corresponding increase in percent positivity. While the number of influenza virus infections may be increasing, the number of respiratory illnesses due to other viruses such as SARS-CoV-2 is increasing more rapidly, resulting in the proportion of respiratory illness due to influenza, or percent positivity, to decrease.
* Reporting delays due to the holiday may have impacted week 52 virologic data; therefore, testing numbers and percent positivity should be interpreted with caution. As additional data are received, we expect to see an increase in the number of positive influenza tests, but we may not see a corresponding increase in percent positivity. While the number of influenza virus infections may be increasing, the number of respiratory illnesses due to other viruses such as SARS-CoV-2 is increasing more rapidly, resulting in the proportion of respiratory illness due to influenza, or percent positivity, to decrease.View Chart Data | View Full Screen Public Health Laboratories
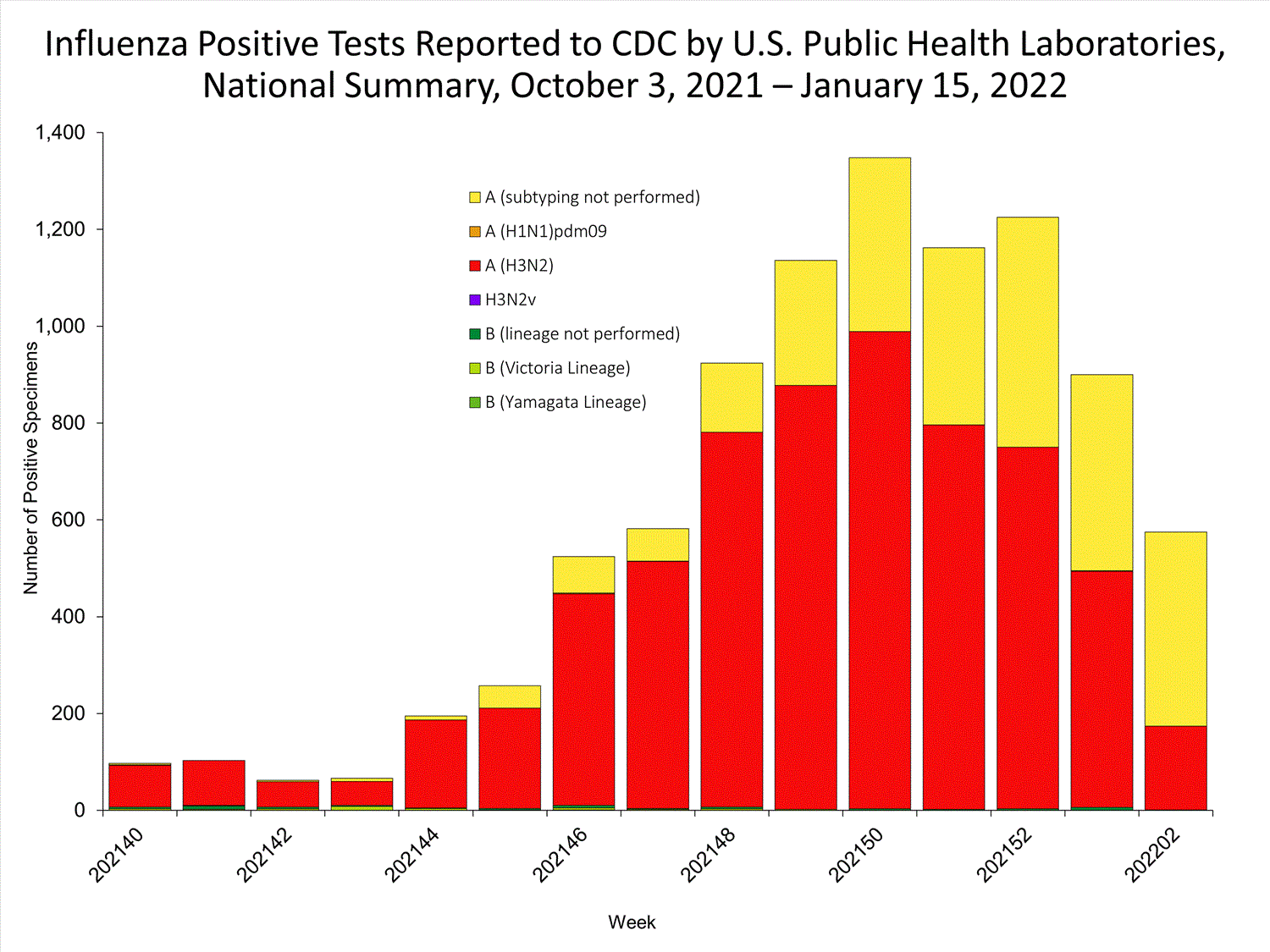
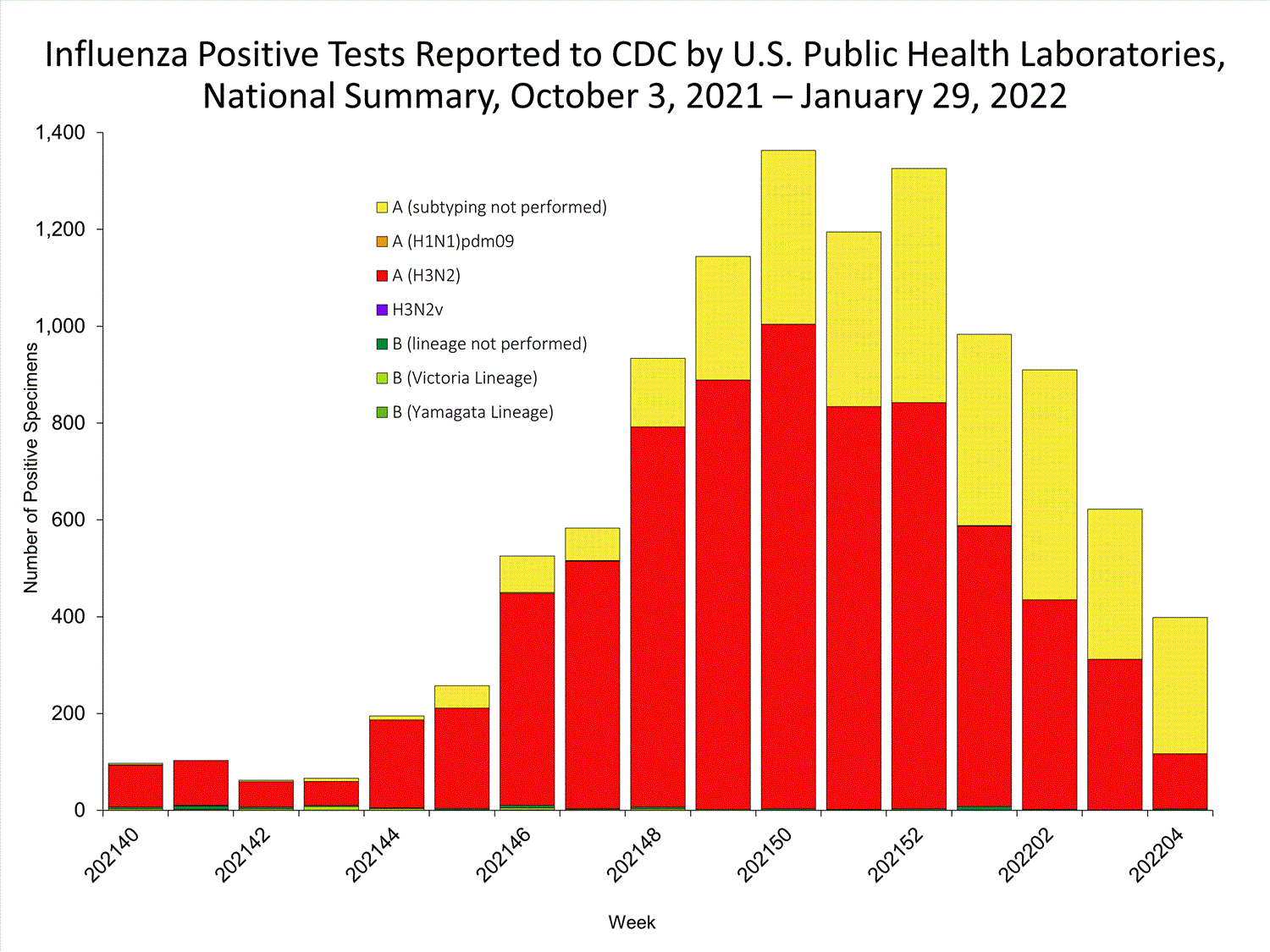
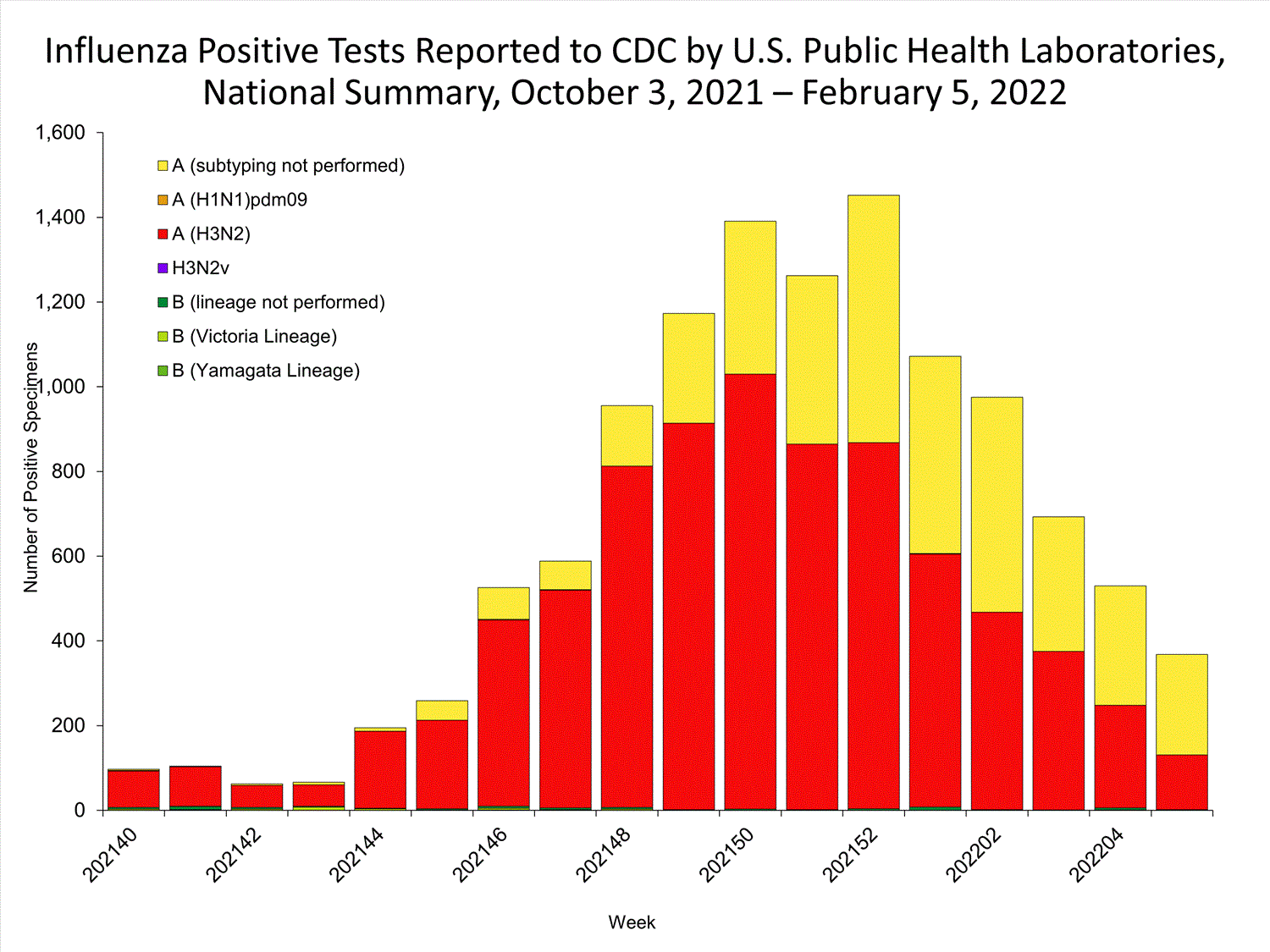
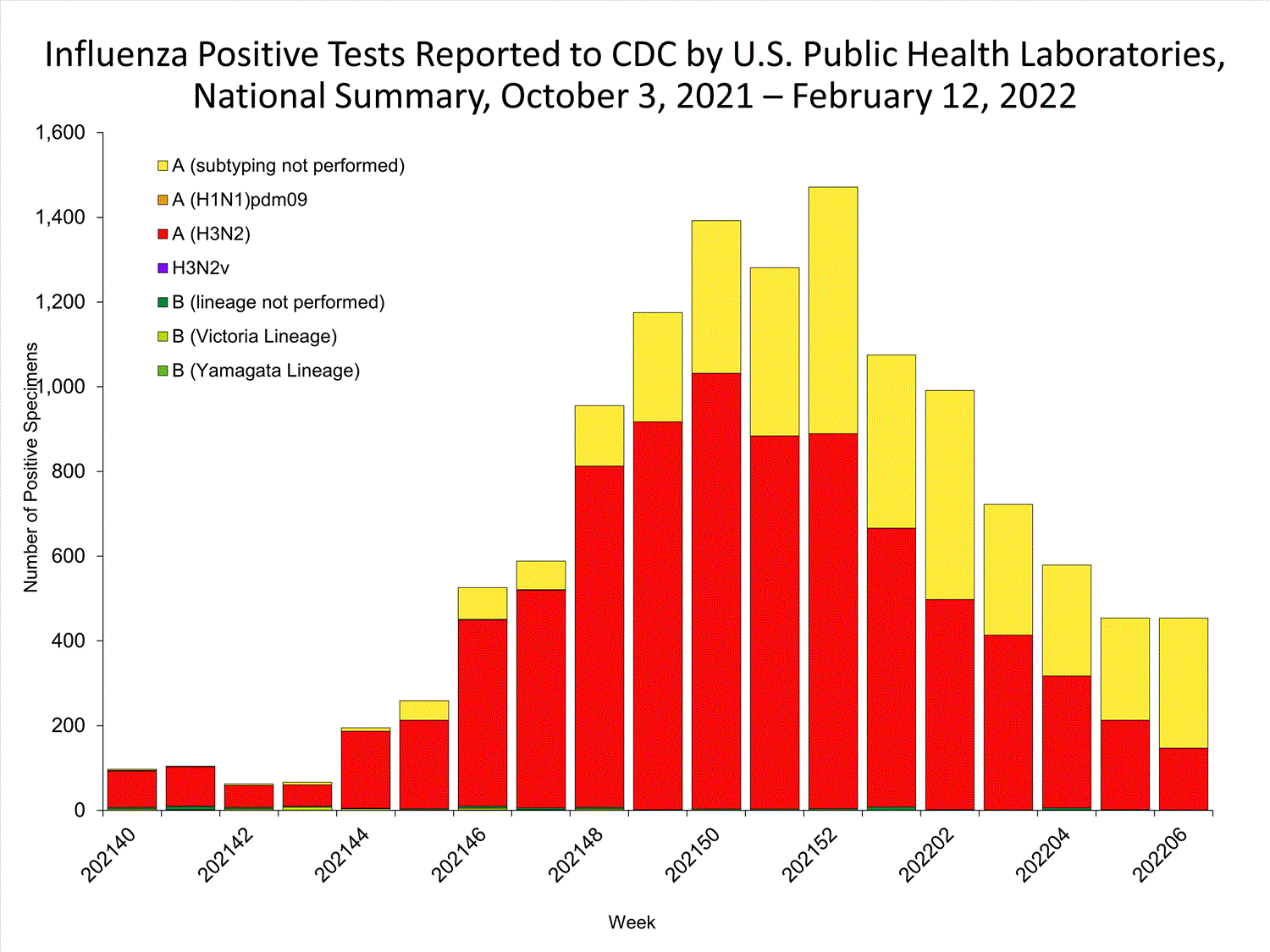
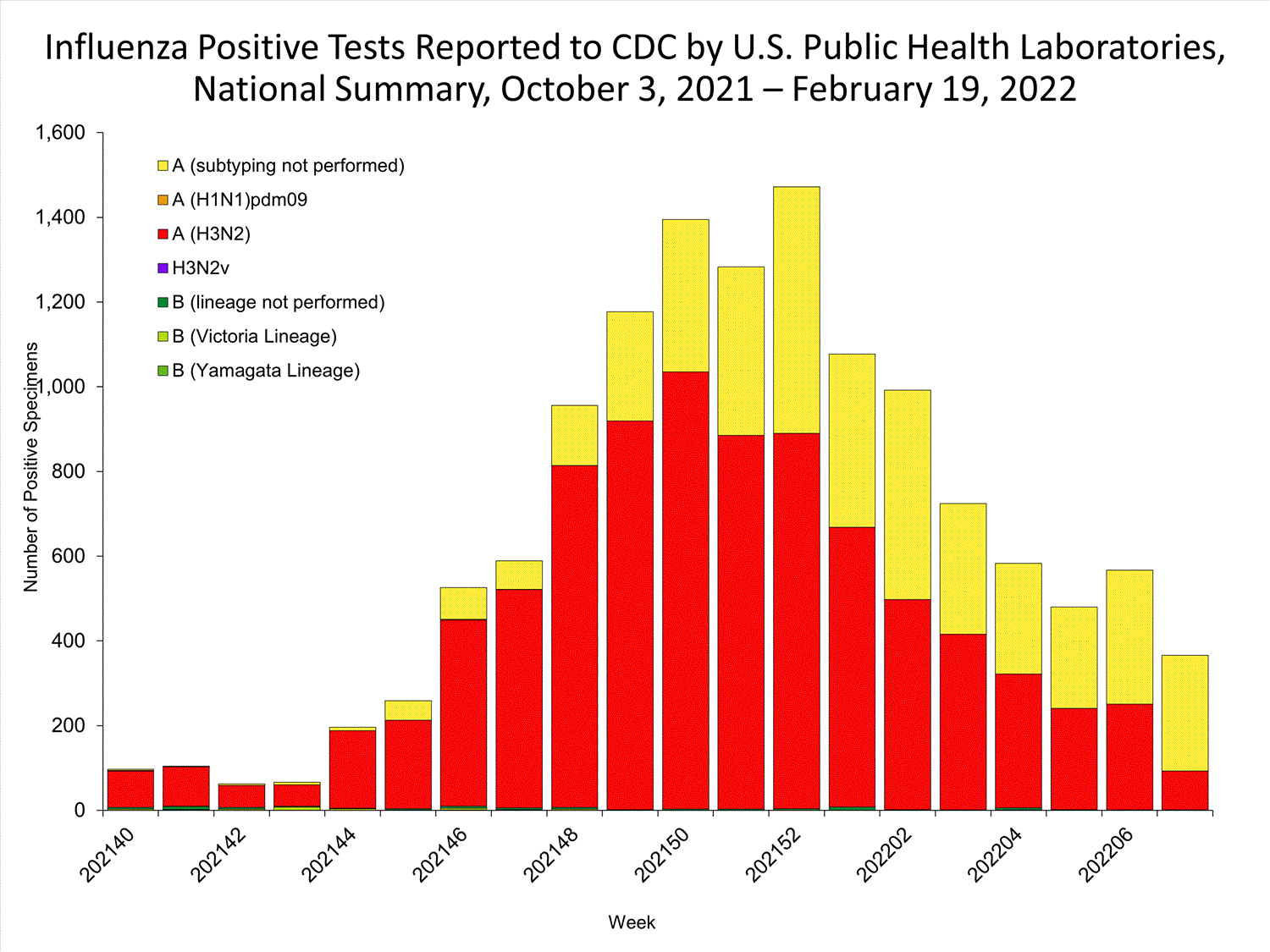
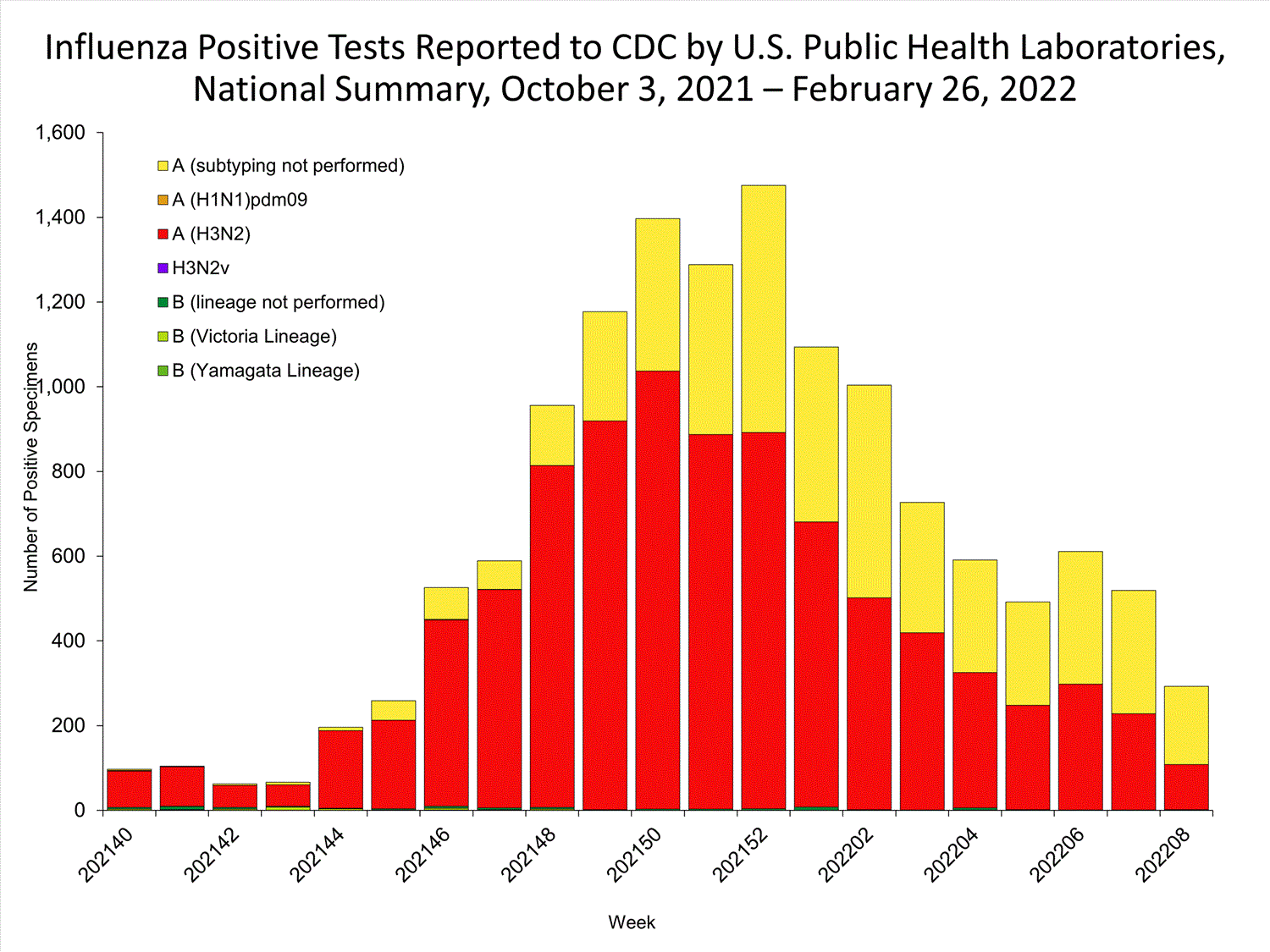
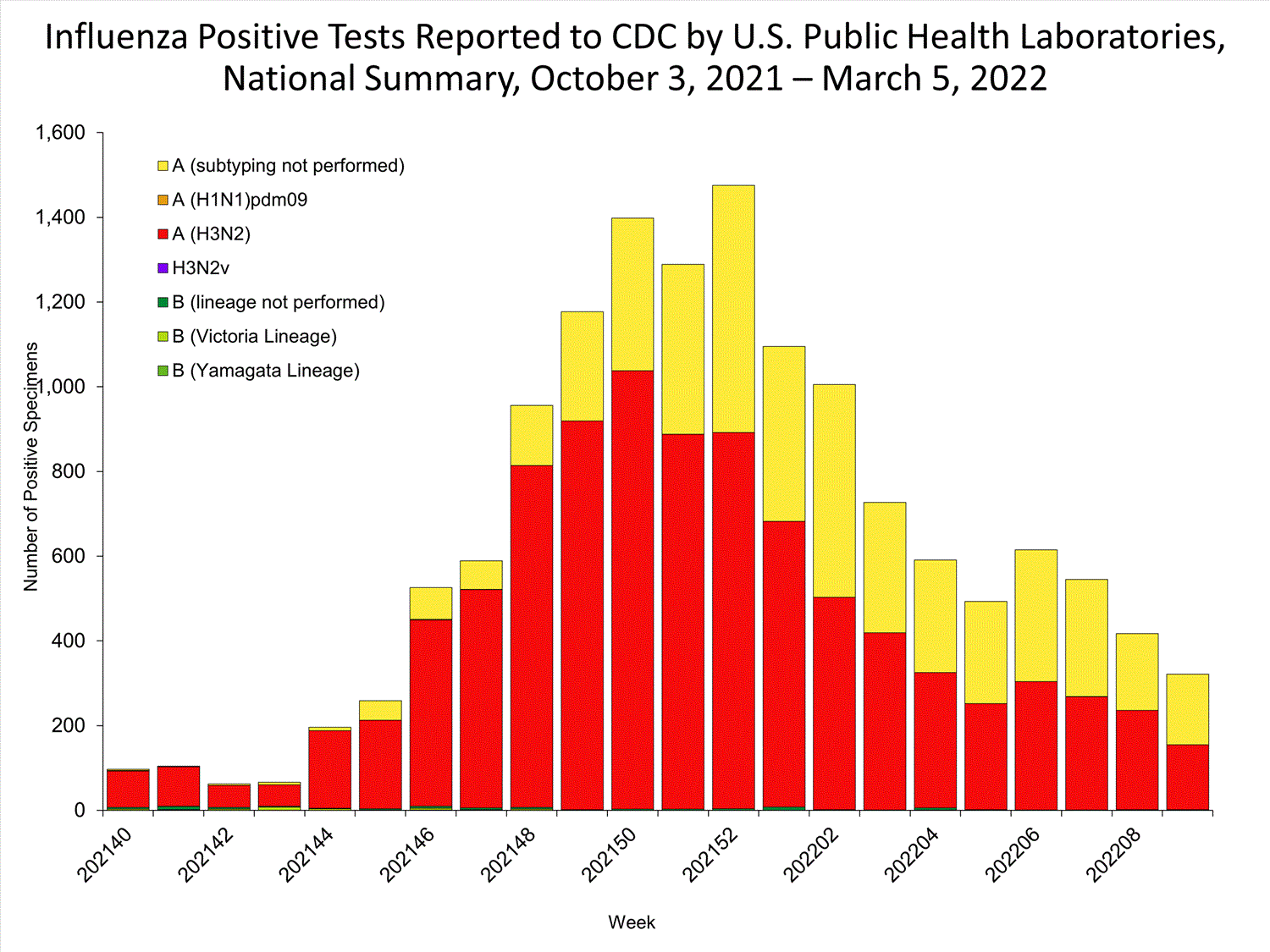
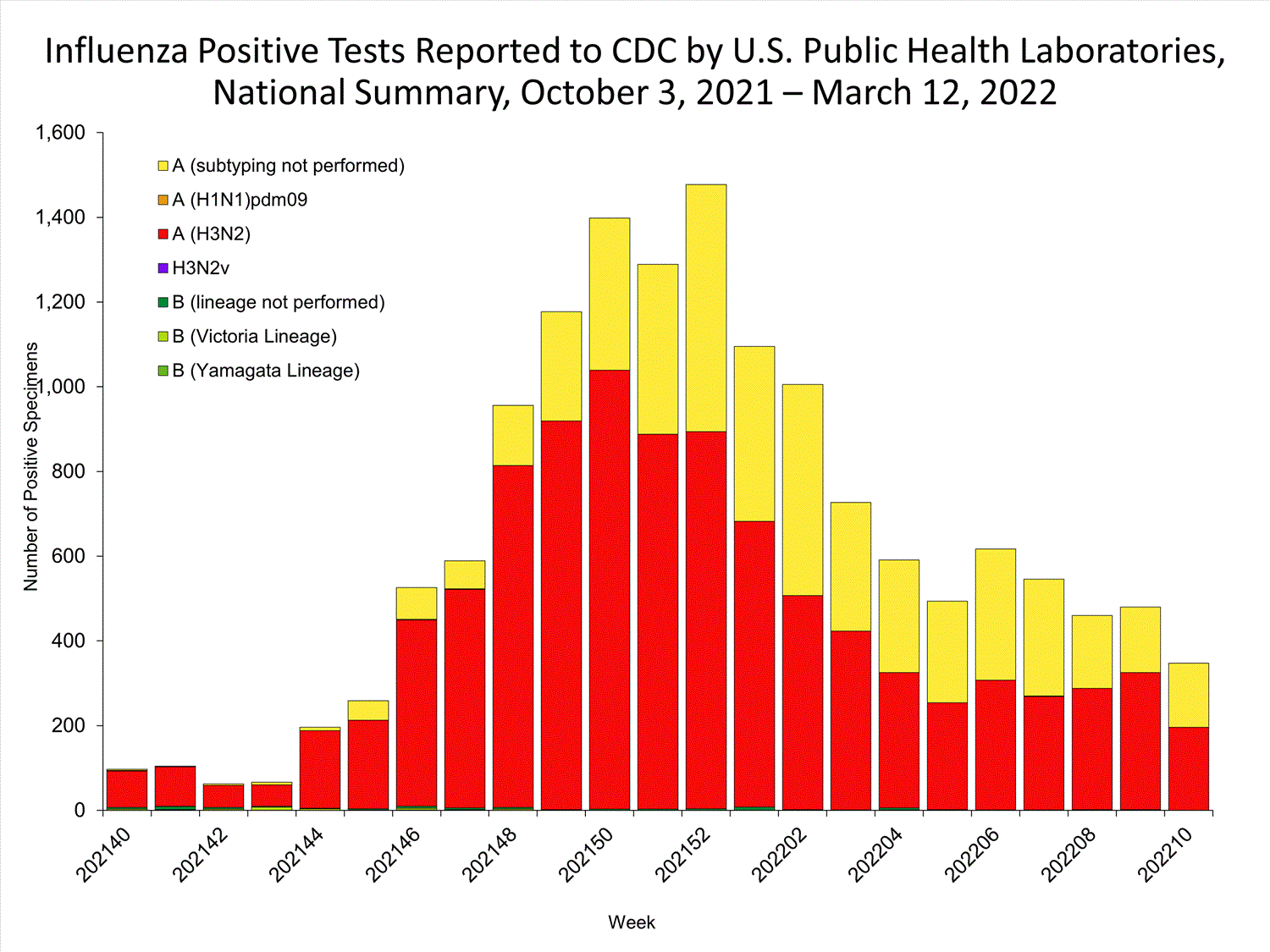
The results of tests performed by public health laboratories nationwide are summarized below. Data from public health laboratories are used to monitor the proportion of circulating viruses that belong to each influenza subtype/lineage.
| No. of specimens tested | 36,233 | 320,638 |
| No. of positive specimens | 785 | 6,658 |
| Positive specimens by type/subtype | ||
| Influenza A | 782 (99.6%) | 6,590 (99.0%) |
| (H1N1)pdm09 | 0 | 4 (0.1%) |
| H3N2 | 377 (100%) | 4,899 (99.9%) |
| H3N2v | 0 | 1 (<0.1%) |
| Subtyping not performed | 405 | 1,686 |
| Influenza B | 3 (0.4%) | 68 (1.0%) |
| Yamagata lineage | 0 | 1 (3.3%) |
| Victoria lineage | 0 | 29 (96.7%) |
| Lineage not performed | 3 | 38 |
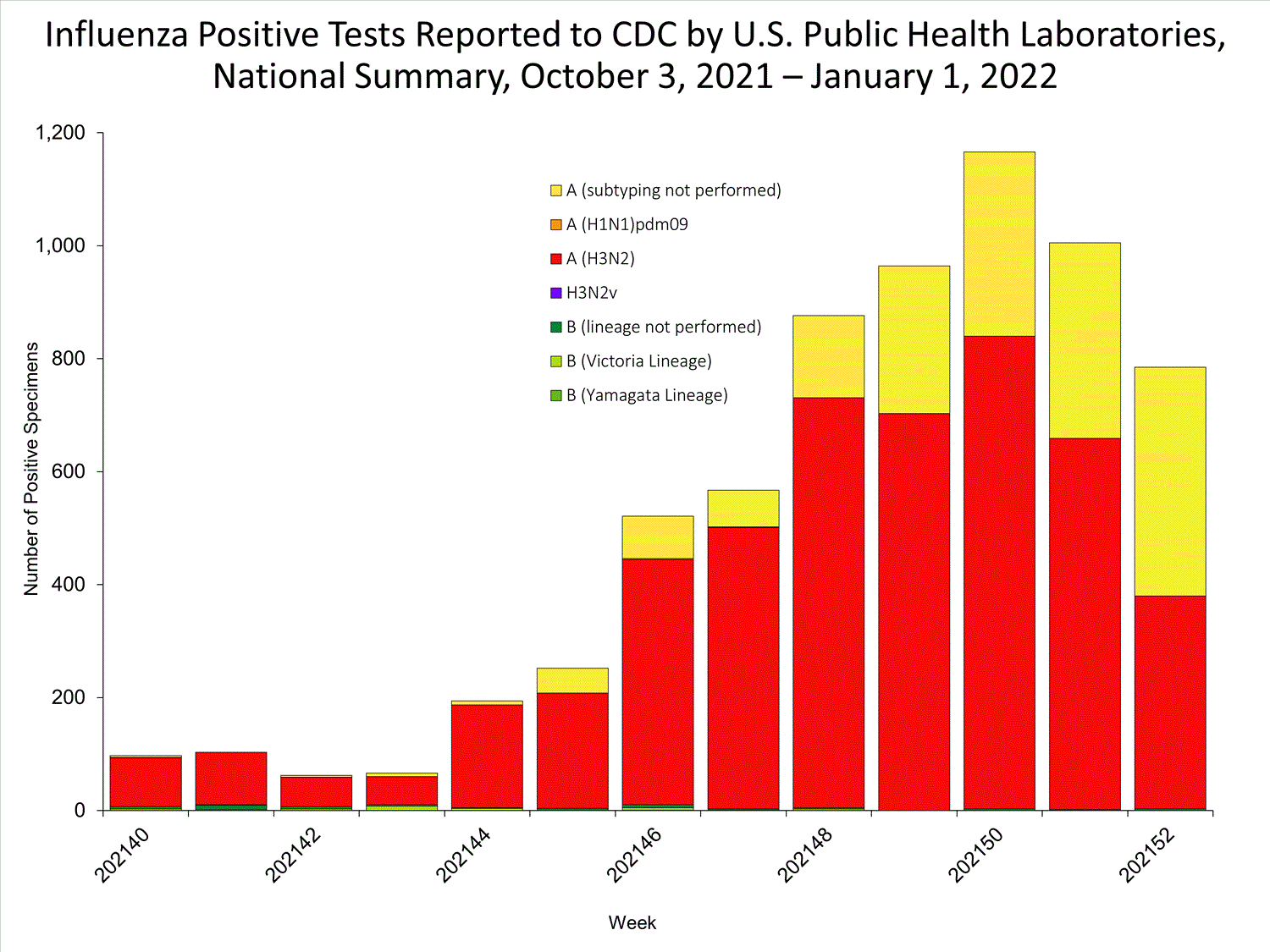 *Reporting delays due to the holiday may have impacted week 52 virologic data; therefore, testing numbers should be interpreted with caution. As additional data are received, we expect to see an increase in the number of positive influenza tests.View Chart Data | View Full Screen
*Reporting delays due to the holiday may have impacted week 52 virologic data; therefore, testing numbers should be interpreted with caution. As additional data are received, we expect to see an increase in the number of positive influenza tests.View Chart Data | View Full Screen Additional virologic surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or Age Data
Influenza Virus Characterization
CDC performs genetic and antigenic characterization of U.S. viruses submitted from state and local public health laboratories using Right Size Roadmap submission guidance. These data are used to compare how similar the currently circulating influenza viruses are to the reference viruses representing viruses contained in the current influenza vaccines. The data are also used to monitor evolutionary changes that continually occur in influenza viruses circulating in humans. CDC also tests susceptibility of circulating influenza viruses to antiviral medications including the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and the PA endonuclease inhibitor baloxavir.
CDC genetically characterized 272 influenza viruses collected since October 3, 2021. While there are little data to date, most of the H3N2 viruses so far are genetically closely related to the vaccine virus, but there are some antigenic differences that have developed as H3N2 viruses have continued to evolve. Virus antigenic data will be reported later this season when a sufficient number of specimens have been tested.
CDC genetically characterized 108 influenza viruses collected October 3, 2021 to present:
| A/H1 | 3 | ||||
| 6B.1A | 3 (100%) | 5a.1 | 2 (67%) | ||
| 5a.2 | 1 (33%) | ||||
| A/H3 | 252 | ||||
| 3C.2a1b | 252 (100%) | 1a | 0 | ||
| 1b | 1 (1%) | ||||
| 2a | 0 | ||||
| 2a.1 | 0 | ||||
| 2a.2 | 251 (99%) | ||||
| 3C.3a | 0 | 3a | 0 | ||
| B/Victoria | 17 | ||||
| V1A | 17 (100%) | V1A | 0 | ||
| V1A.1 | 0 | ||||
| V1A.3 | 10 (59%) | ||||
| V1A.3a | 0 | ||||
| V1A.3a.1 | 0 | ||||
| V1A.3a.2 | 7 (41%) | ||||
| B/Yamagata | 0 | ||||
| Y3 | 0 |
Viruses collected in the United States since October 3, 2021, were tested for antiviral susceptibility as follows:
| Neuraminidase Inhibitors |
|||||||
| Oseltamivir | Viruses Tested |
269 | 3 | 250 | 16 | 0 | |
| Reduced Inhibition |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| Highly Reduced Inhibition |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| Peramivir | Viruses Tested |
269 | 3 | 250 | 16 | 0 | |
| Reduced Inhibition |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| Highly Reduced Inhibition |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| Zanamivir | Viruses Tested |
269 | 3 | 250 | 16 | 0 | |
| Reduced Inhibition |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| Highly Reduced Inhibition |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) | ||
| PA Cap-Dependent Endonuclease Inhibitor | Baloxavir | Viruses Tested |
259 | 3 | 240 | 16 | 0 |
| Reduced Susceptibility |
(0.0%) | (0.0%) | (0.0%) | (0.0%) | (0.0%) |
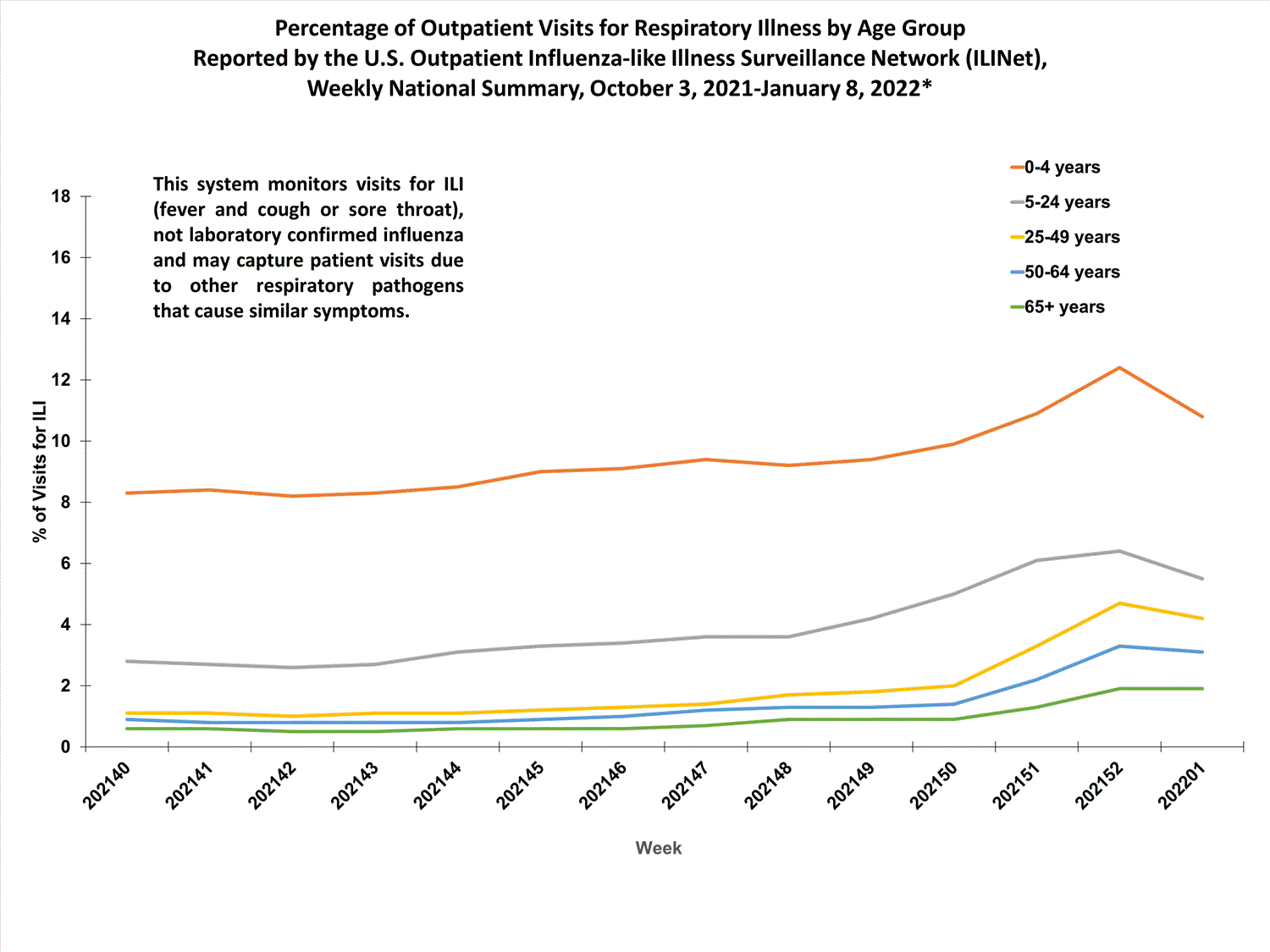
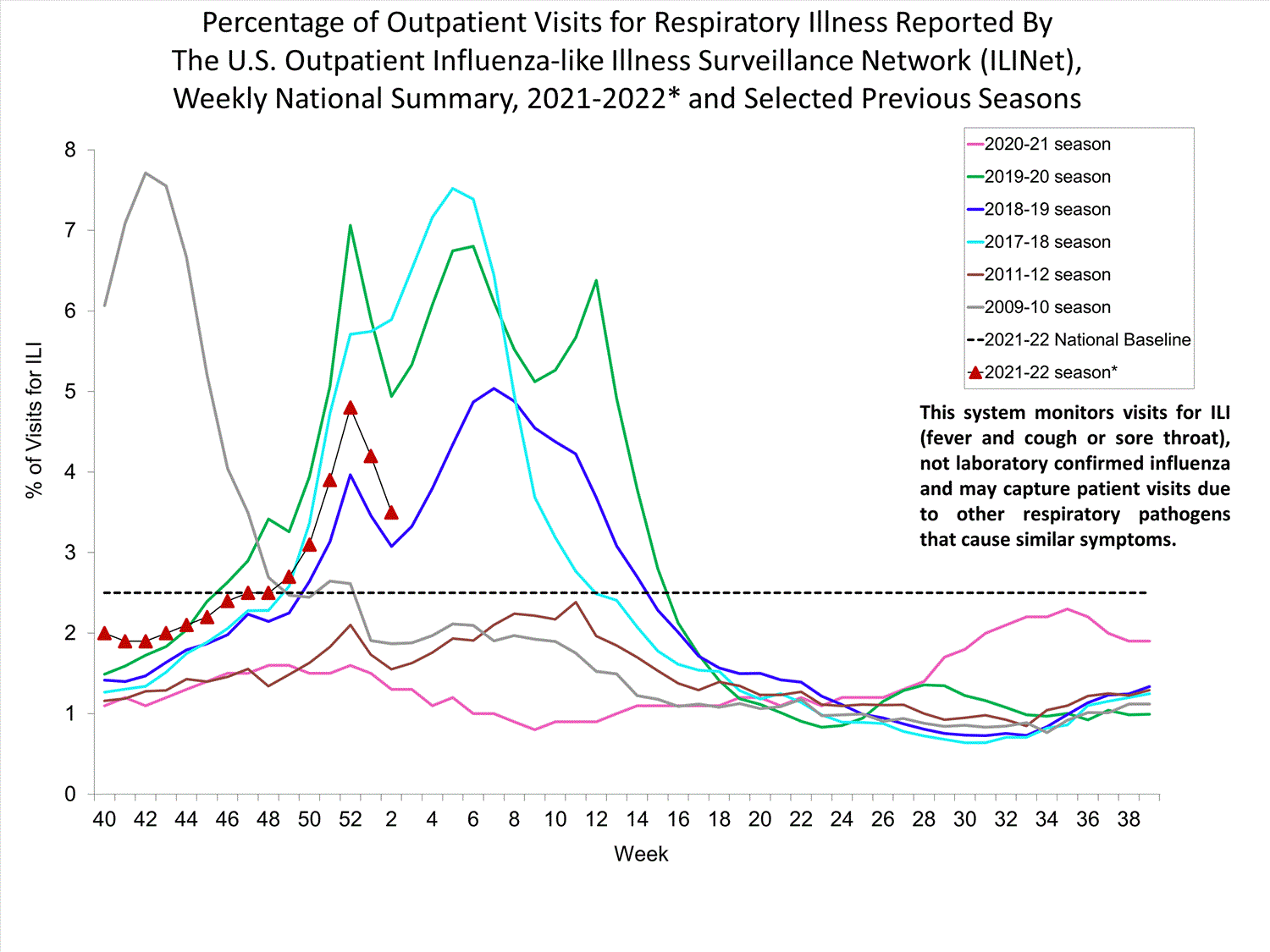
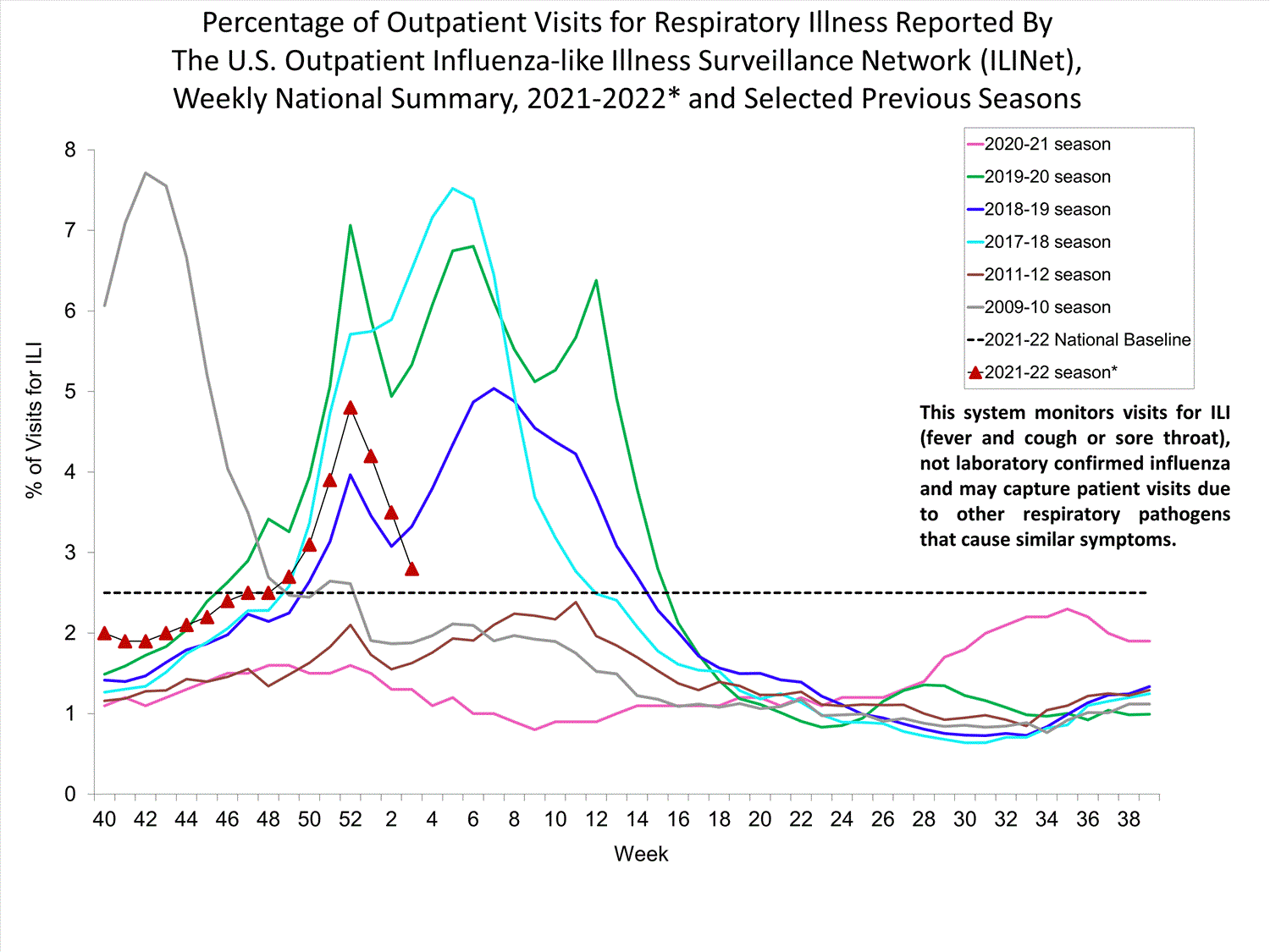
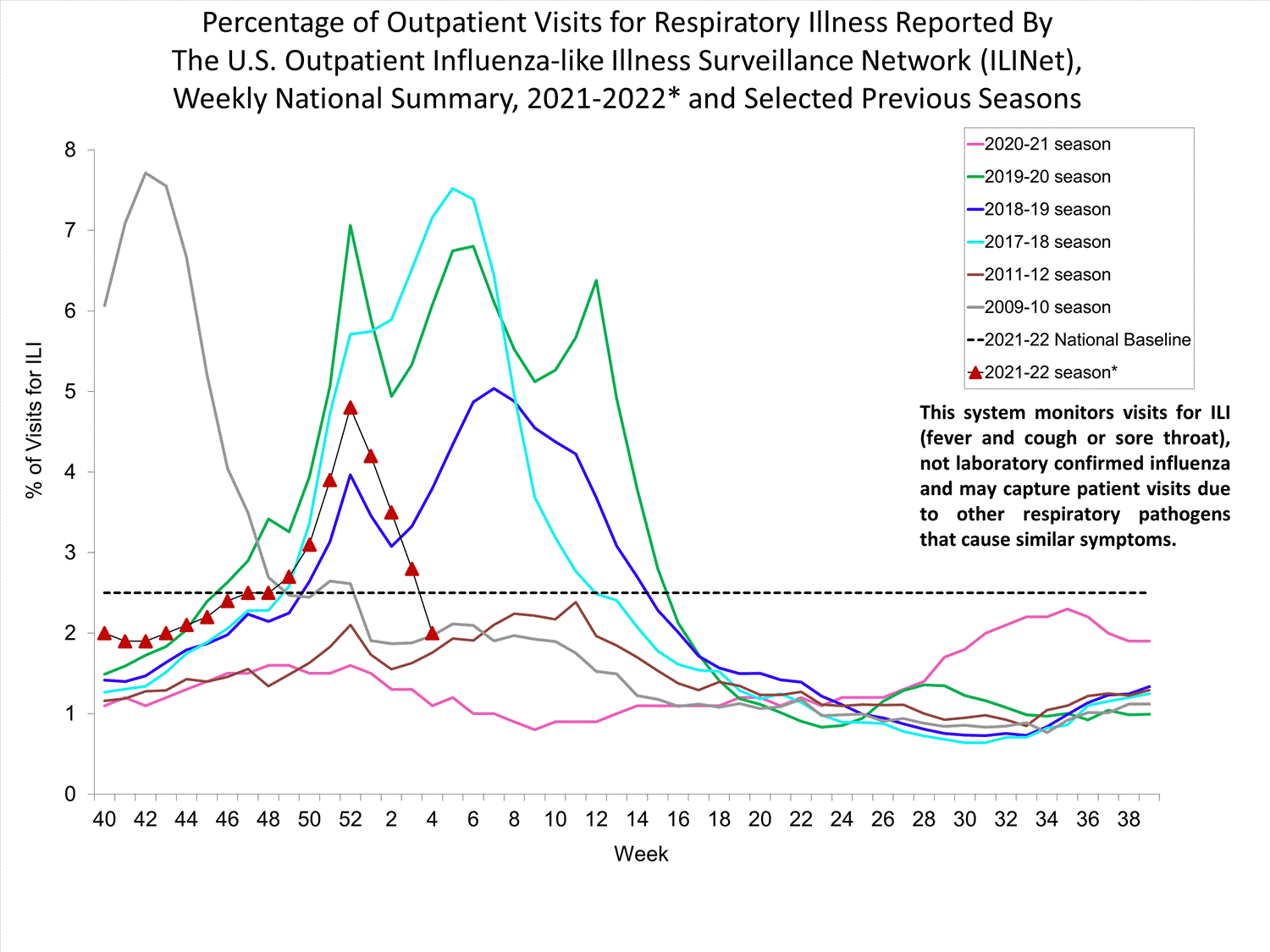
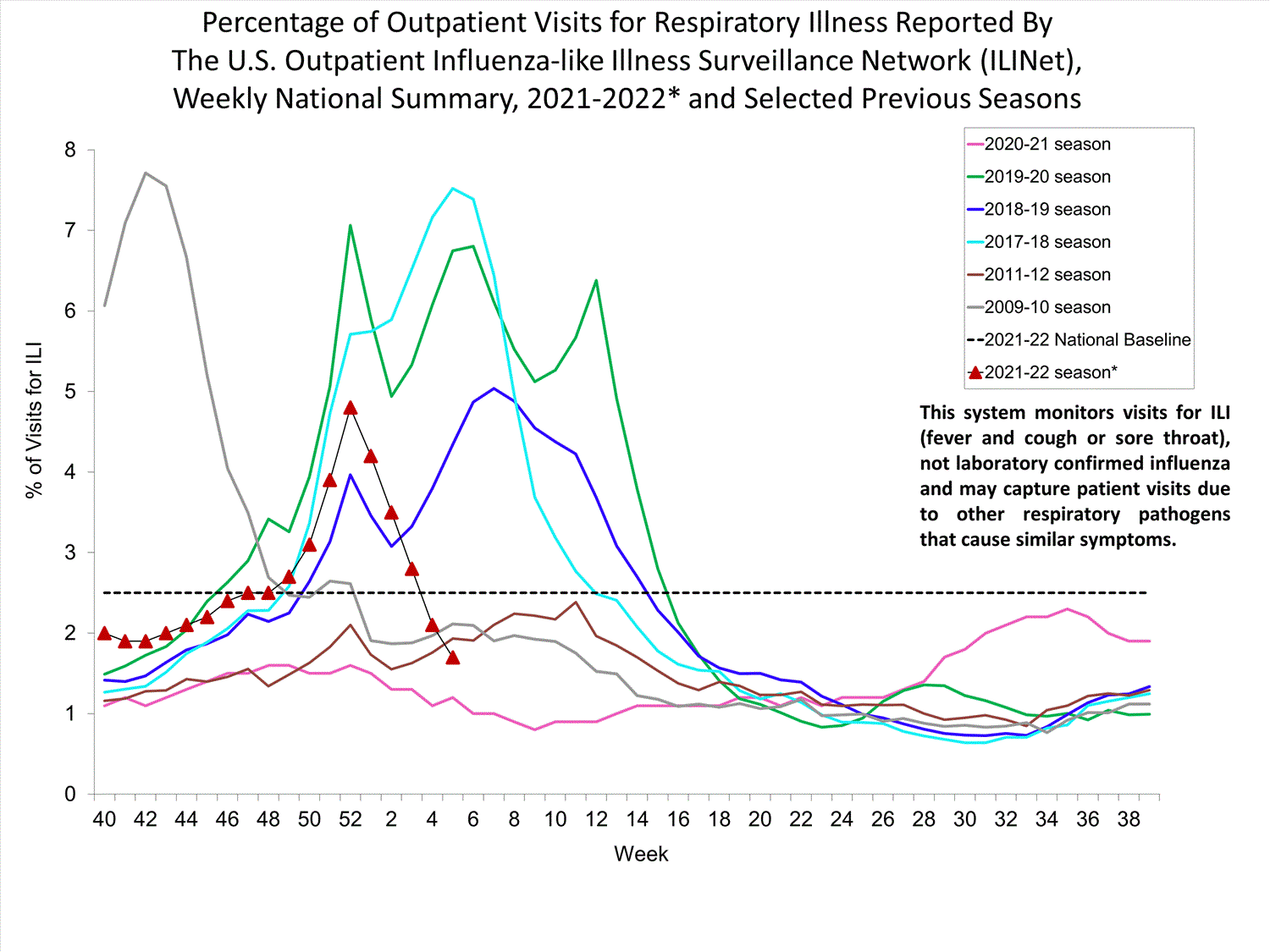
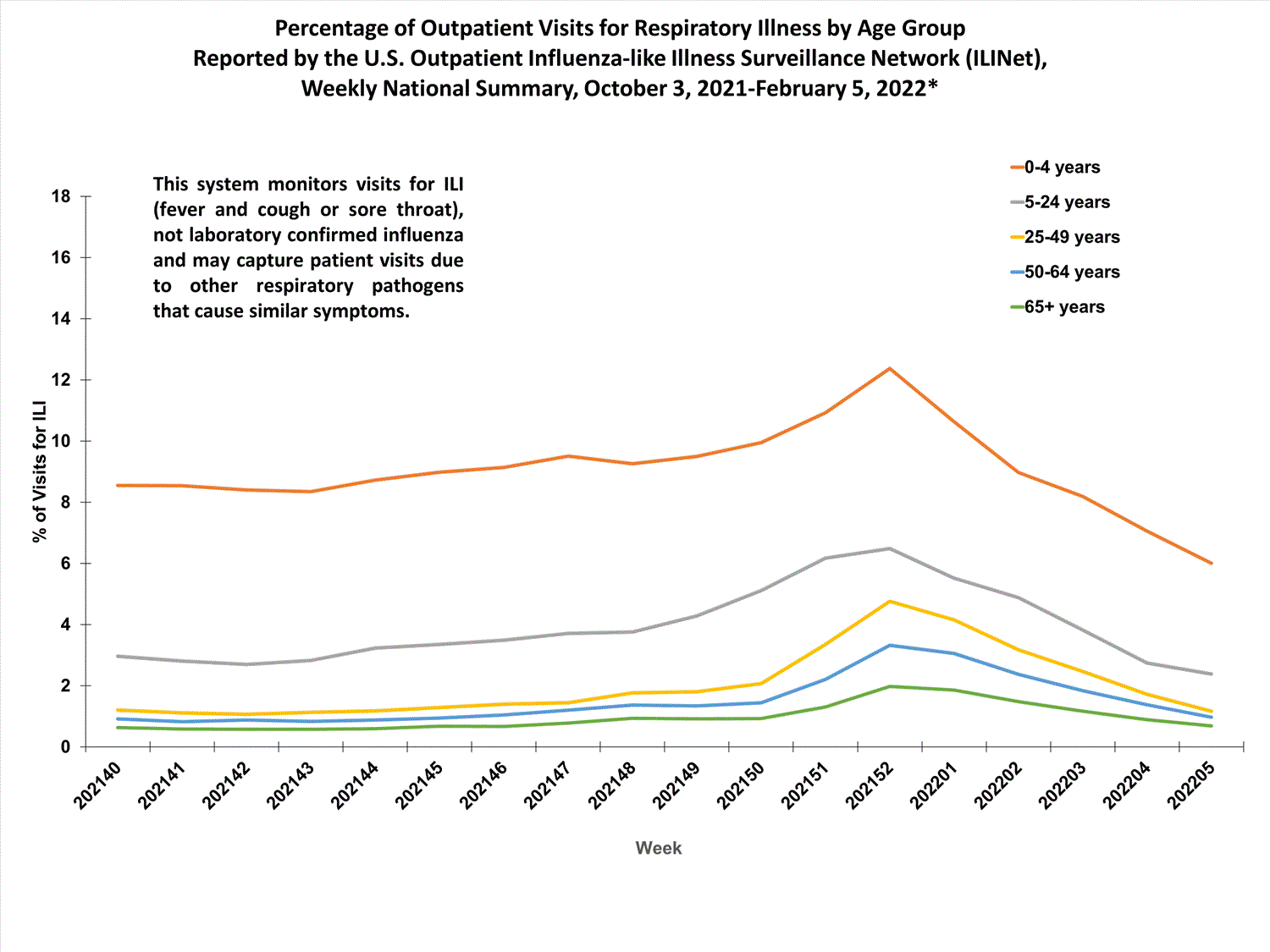
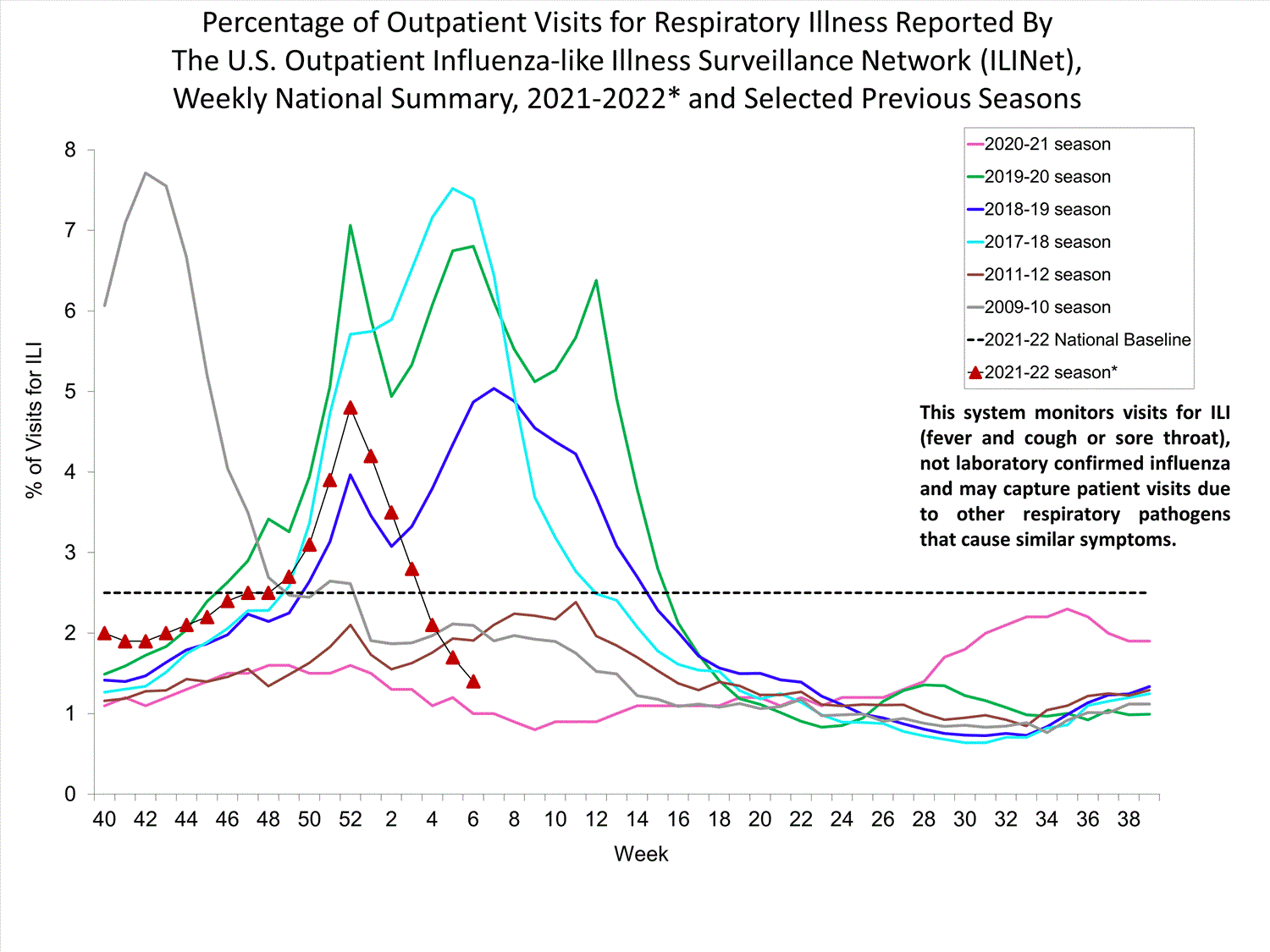
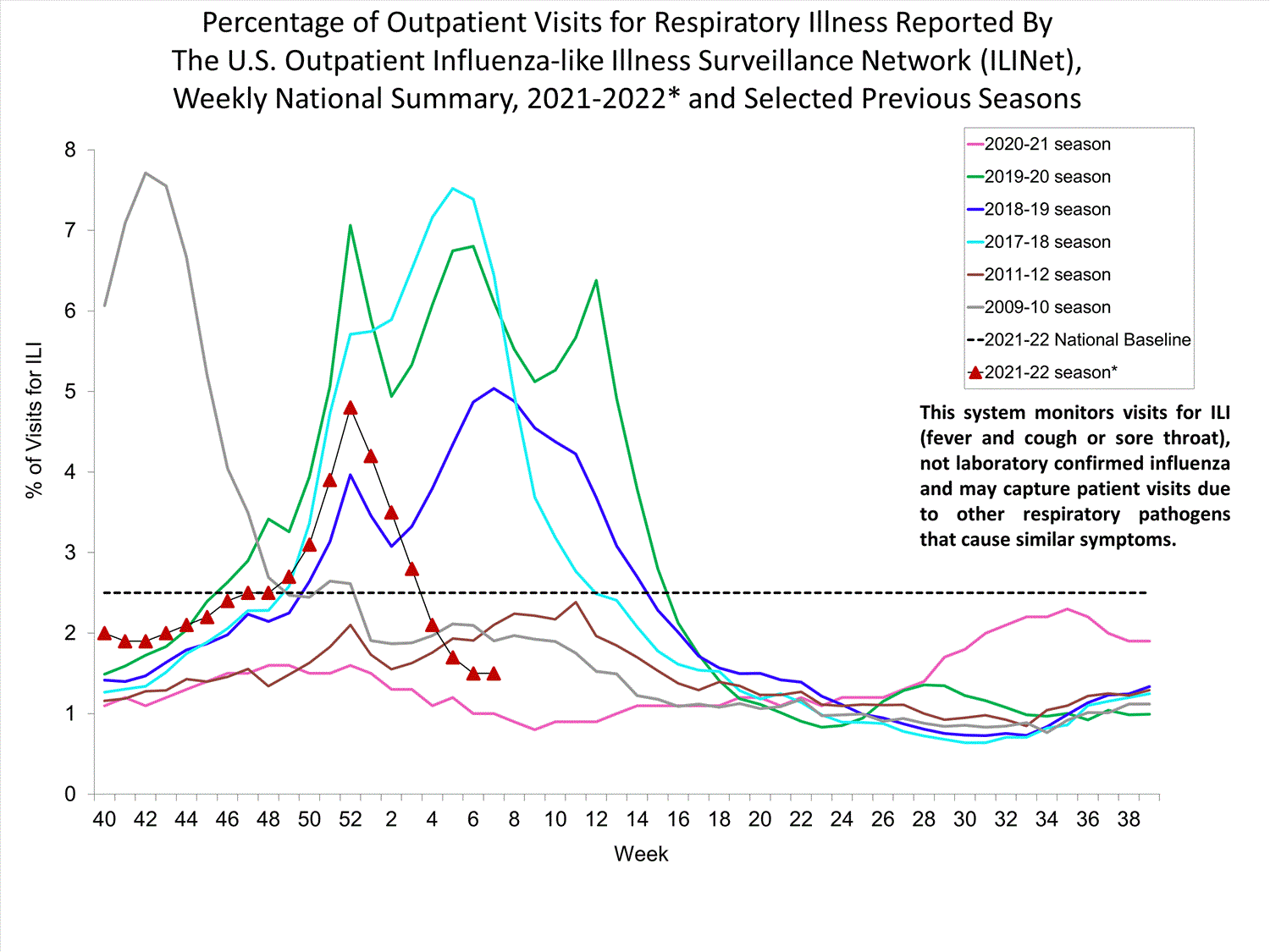
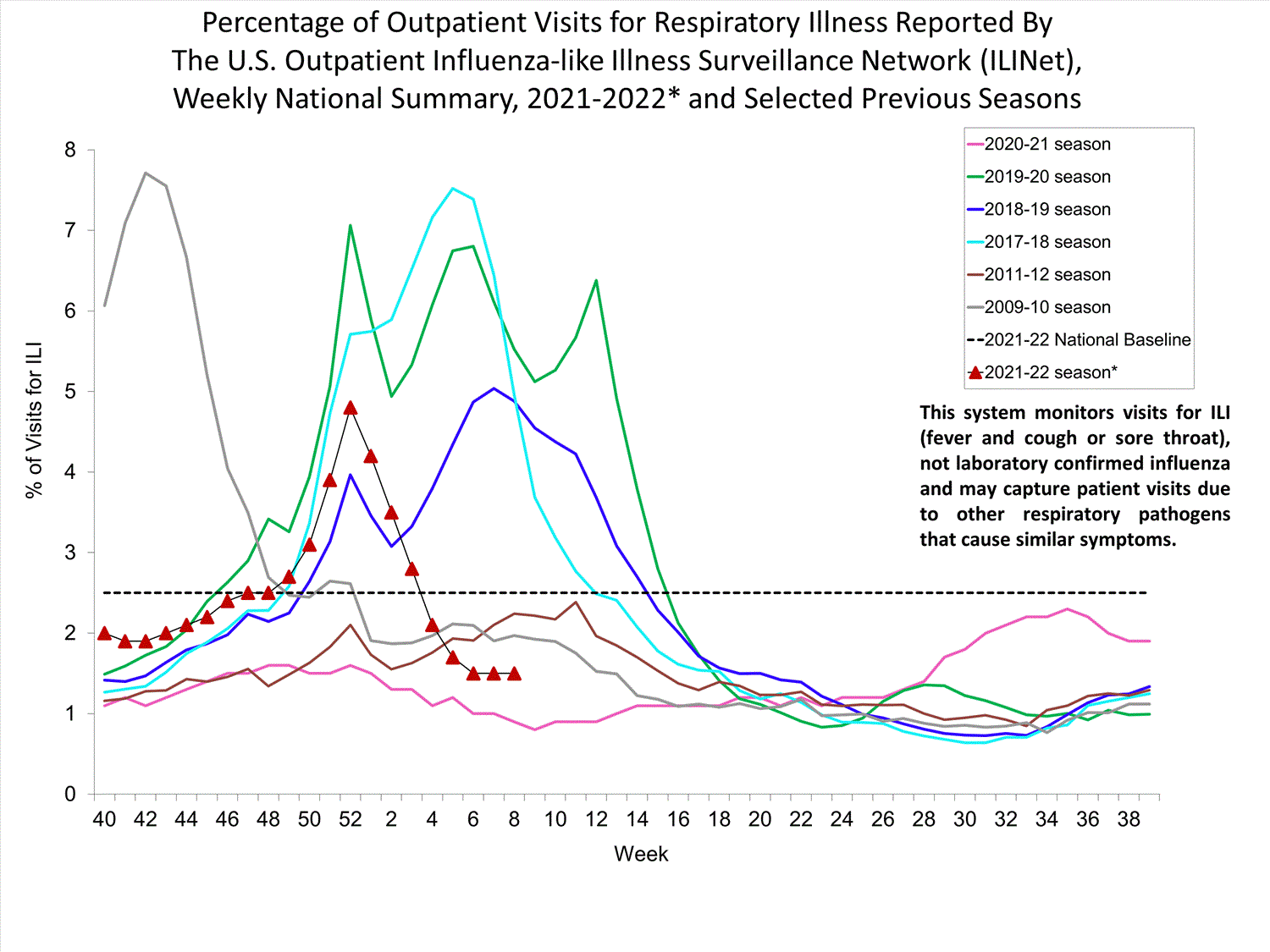
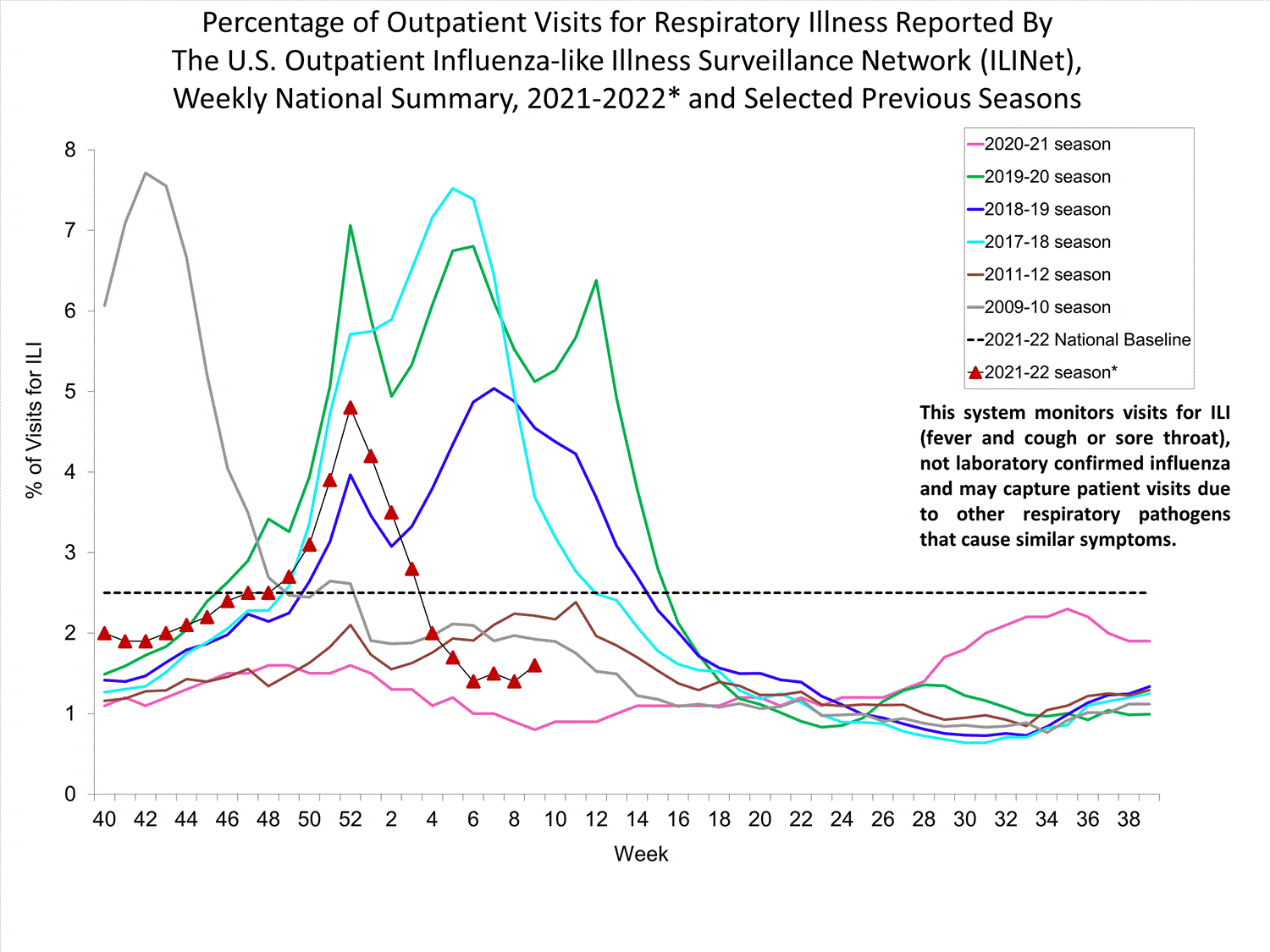
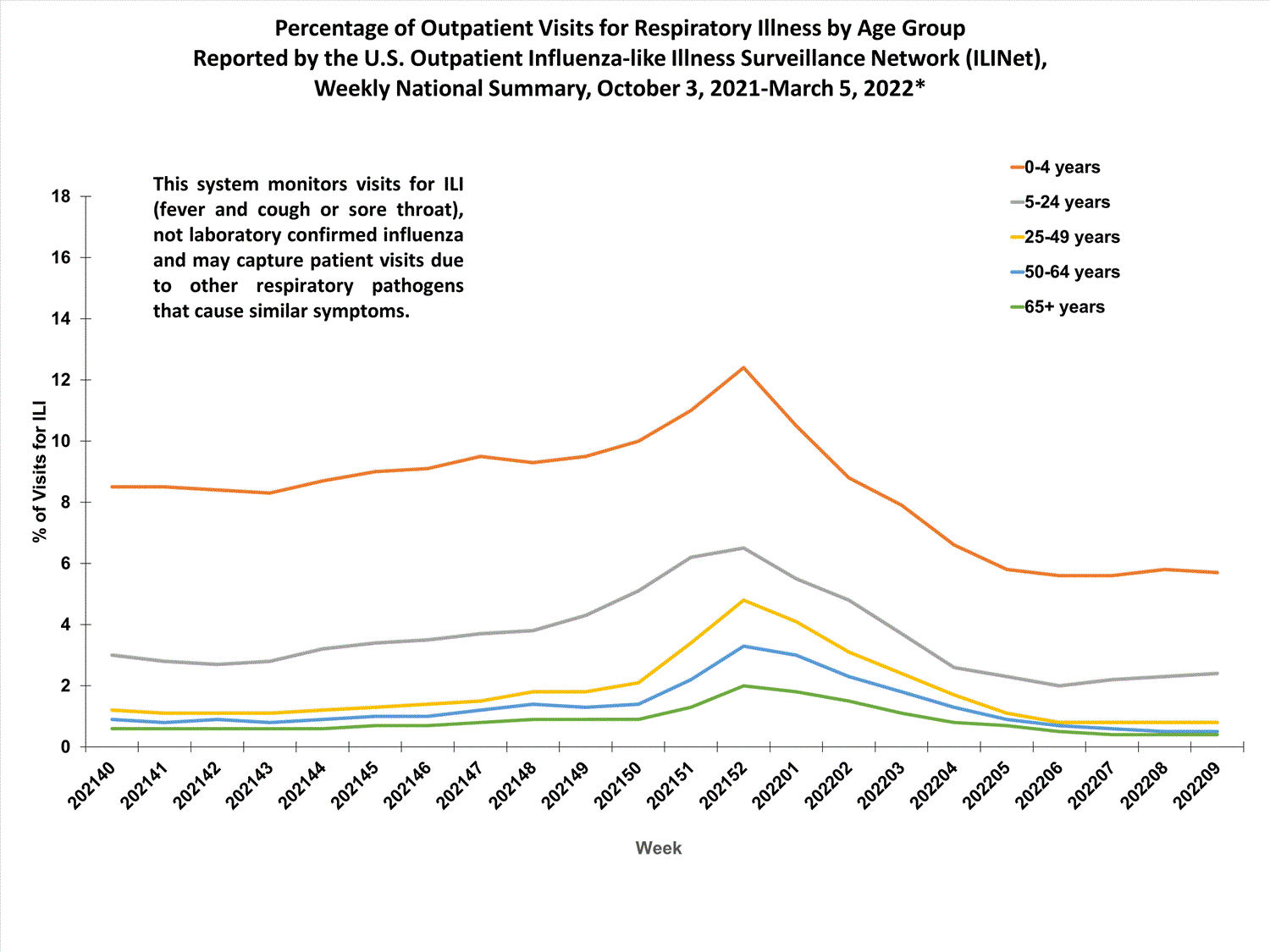
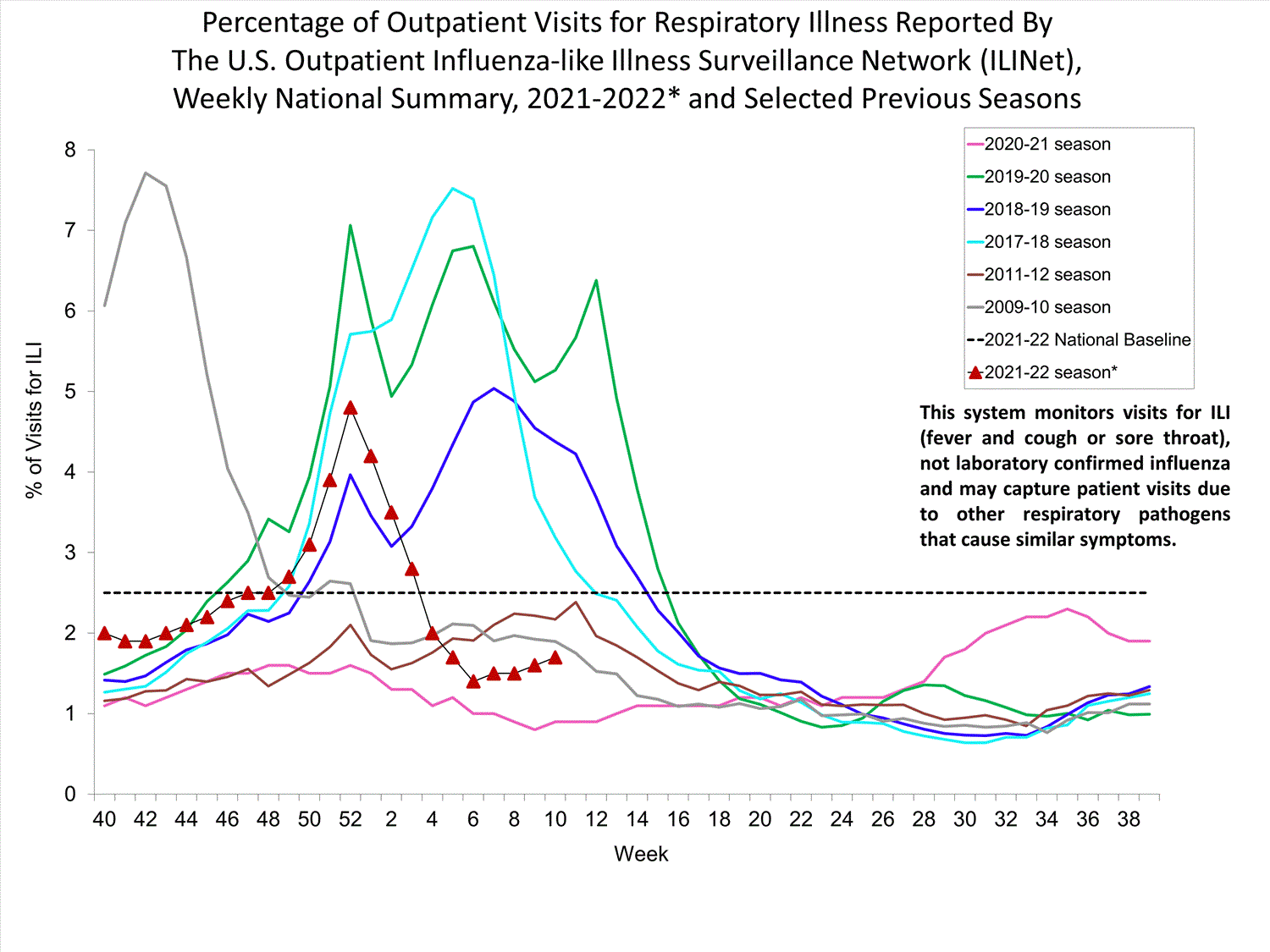
Outpatient Respiratory Illness Surveillance
The U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) monitors outpatient visits for influenza-like illness [ILI (fever plus cough or sore throat)], not laboratory-confirmed influenza, and will therefore capture respiratory illness visits due to infection with any pathogen that can present with similar symptoms such as influenza, SARS-CoV-2, and RSV. Due to the COVID-19 pandemic, health care-seeking behaviors have changed, and people may be accessing the health care system in alternative settings not captured as a part of ILINet or at a different point in their illness than they might have before the pandemic. Therefore, it is important to evaluate syndromic surveillance data, including that from ILINet, in the context of other sources of surveillance data to obtain a complete and accurate picture of influenza, SARS-CoV-2, and other respiratory virus activity. CDC is tracking the COVID-19 pandemic in a weekly publication called COVID Data Tracker Weekly Review. Information about other respiratory virus activity can be found on CDC’s National Respiratory and Enteric Virus Surveillance System (NREVSS) website.
Outpatient Respiratory Illness Visits
Nationwide, during week 52, 4.8% of patient visits reported through ILINet were due to respiratory illness that included fever plus a cough or sore throat, also referred to as ILI. This percentage is above the national baseline. All 10 HHS regions are above their region-specific baselines. Multiple respiratory viruses are co-circulating, and the relative contribution of influenza virus infection to ILI varies by location.
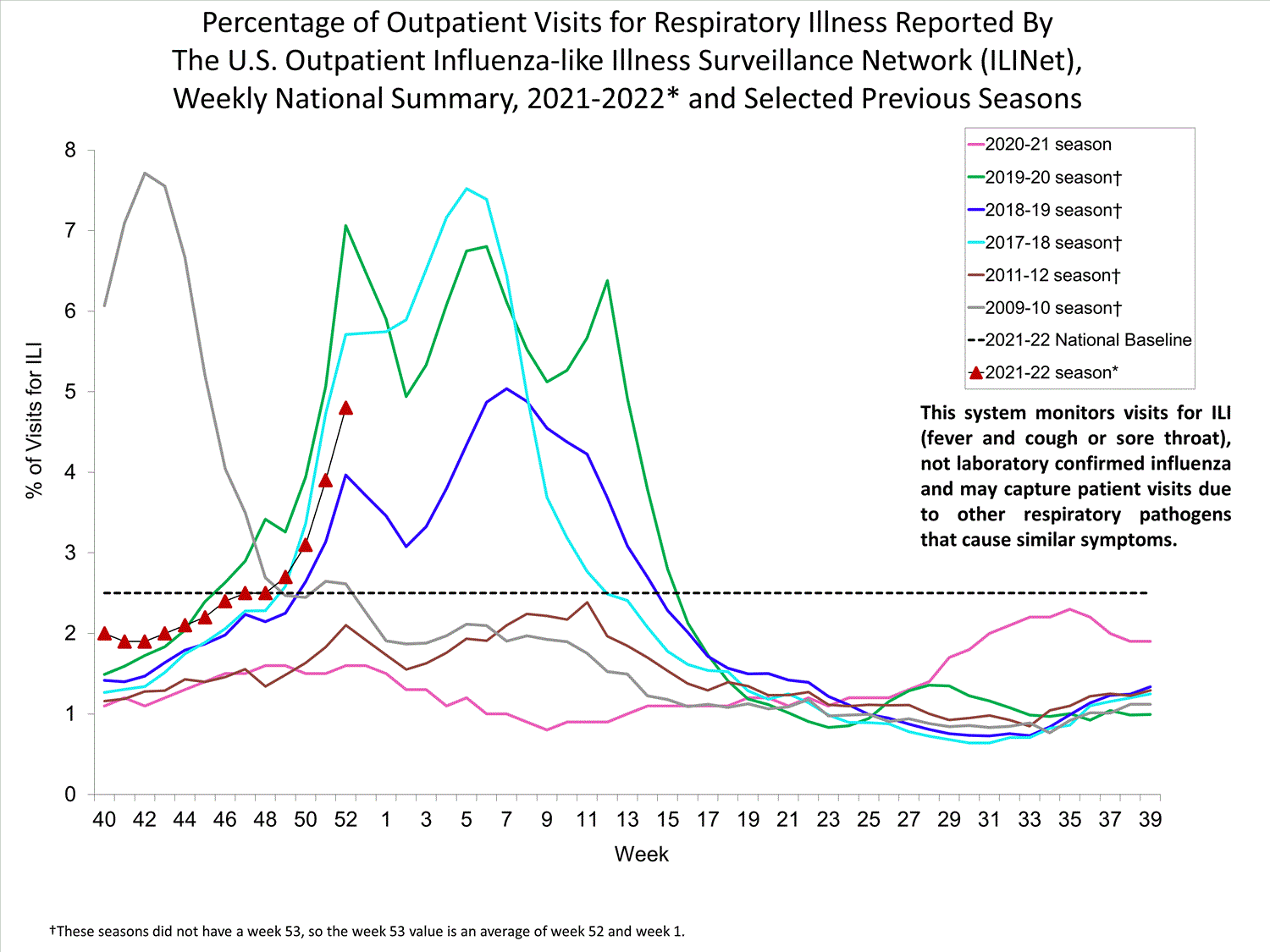
* Effective October 3, 2021 (week 40), the ILI definition (fever plus cough or sore throat) no longer includes “without a known cause other than influenza.”
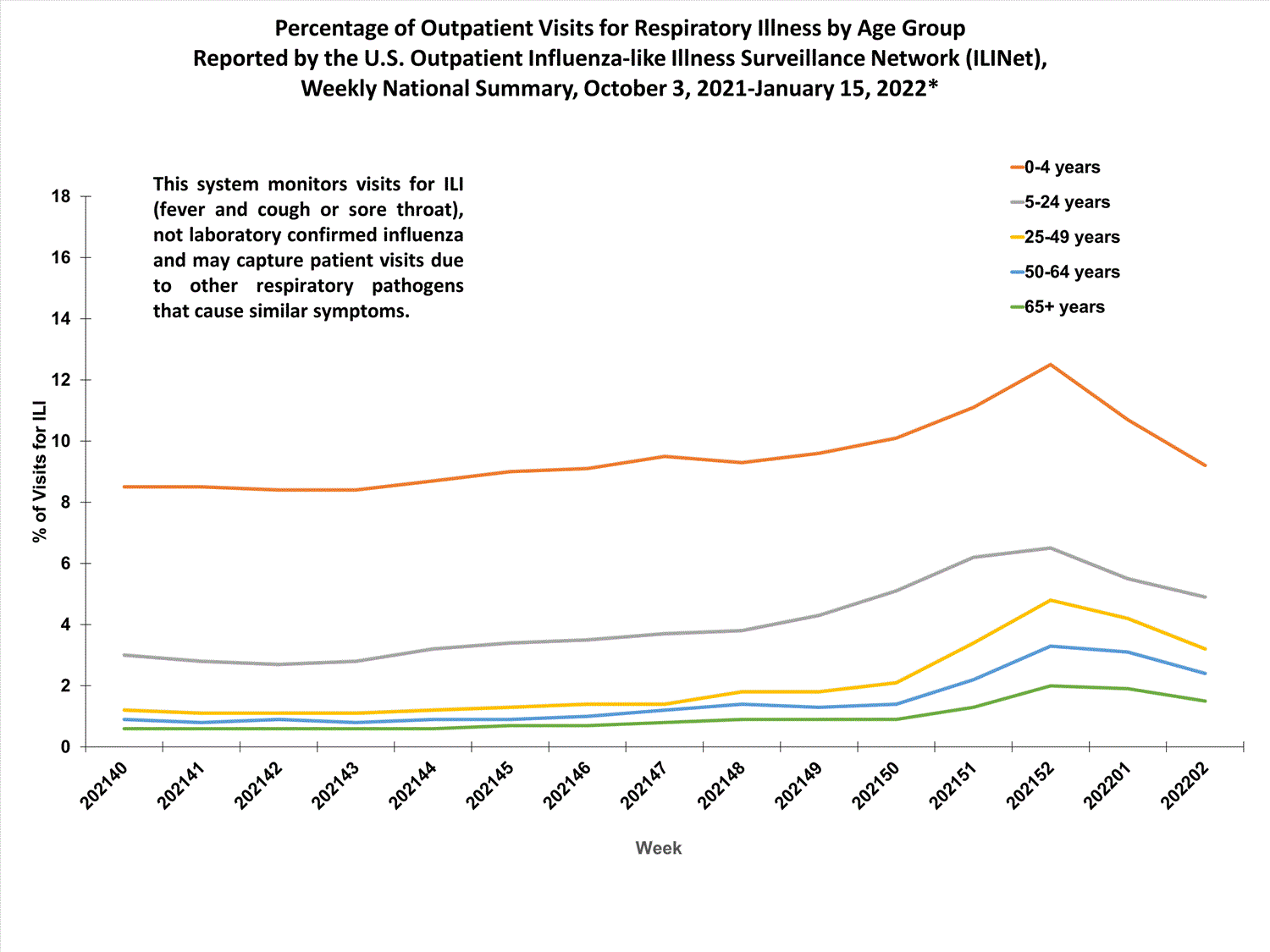
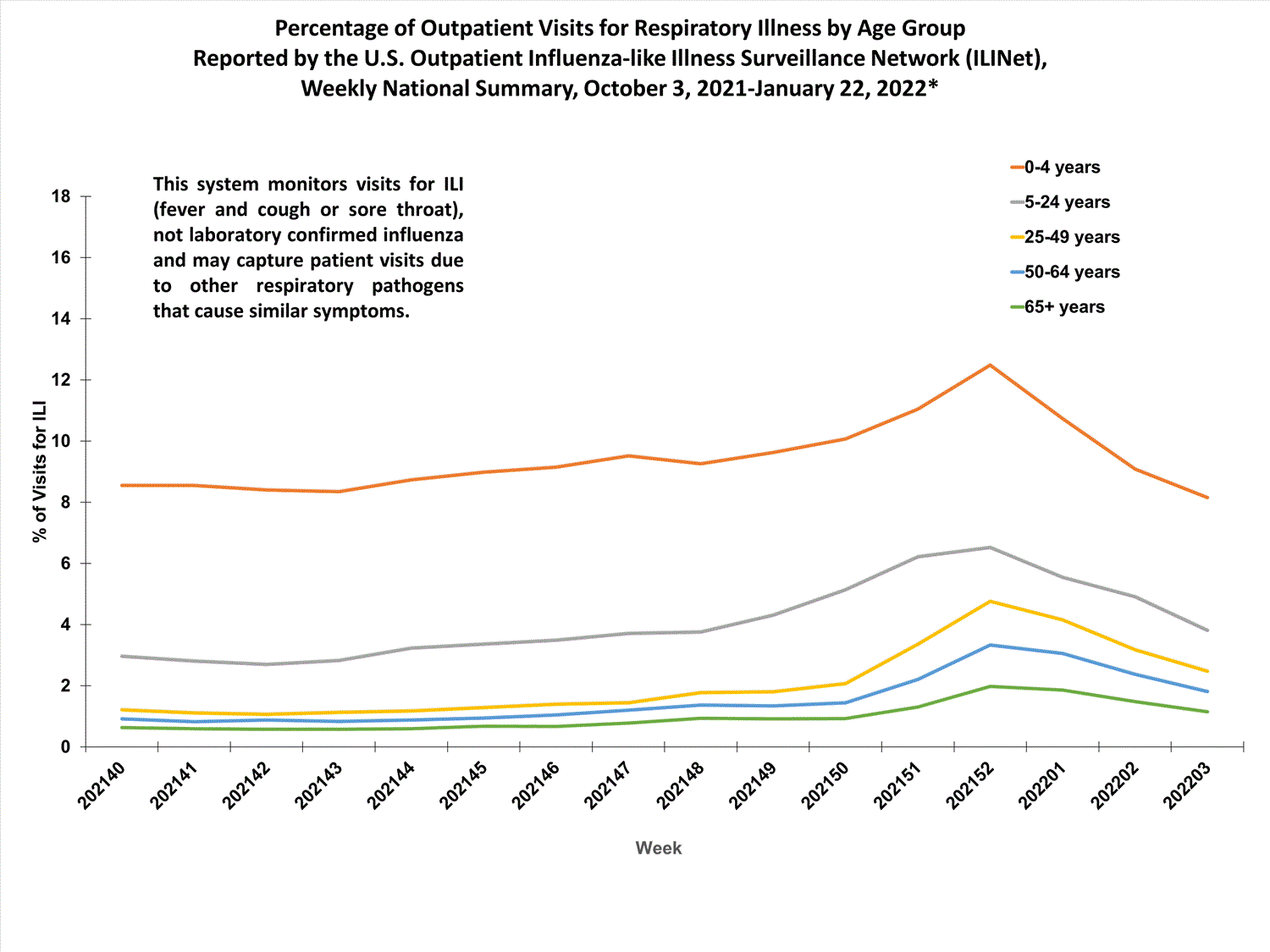
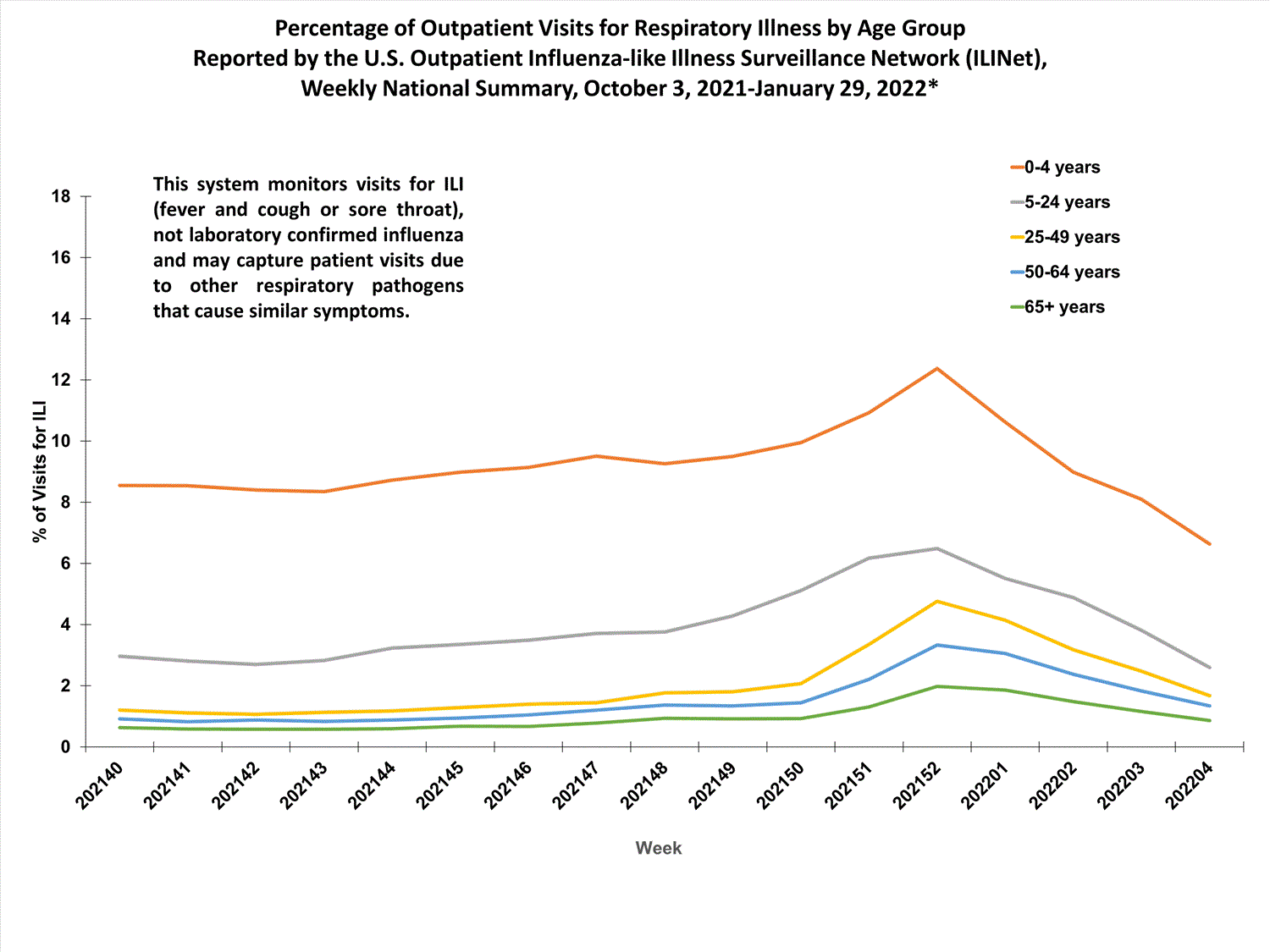
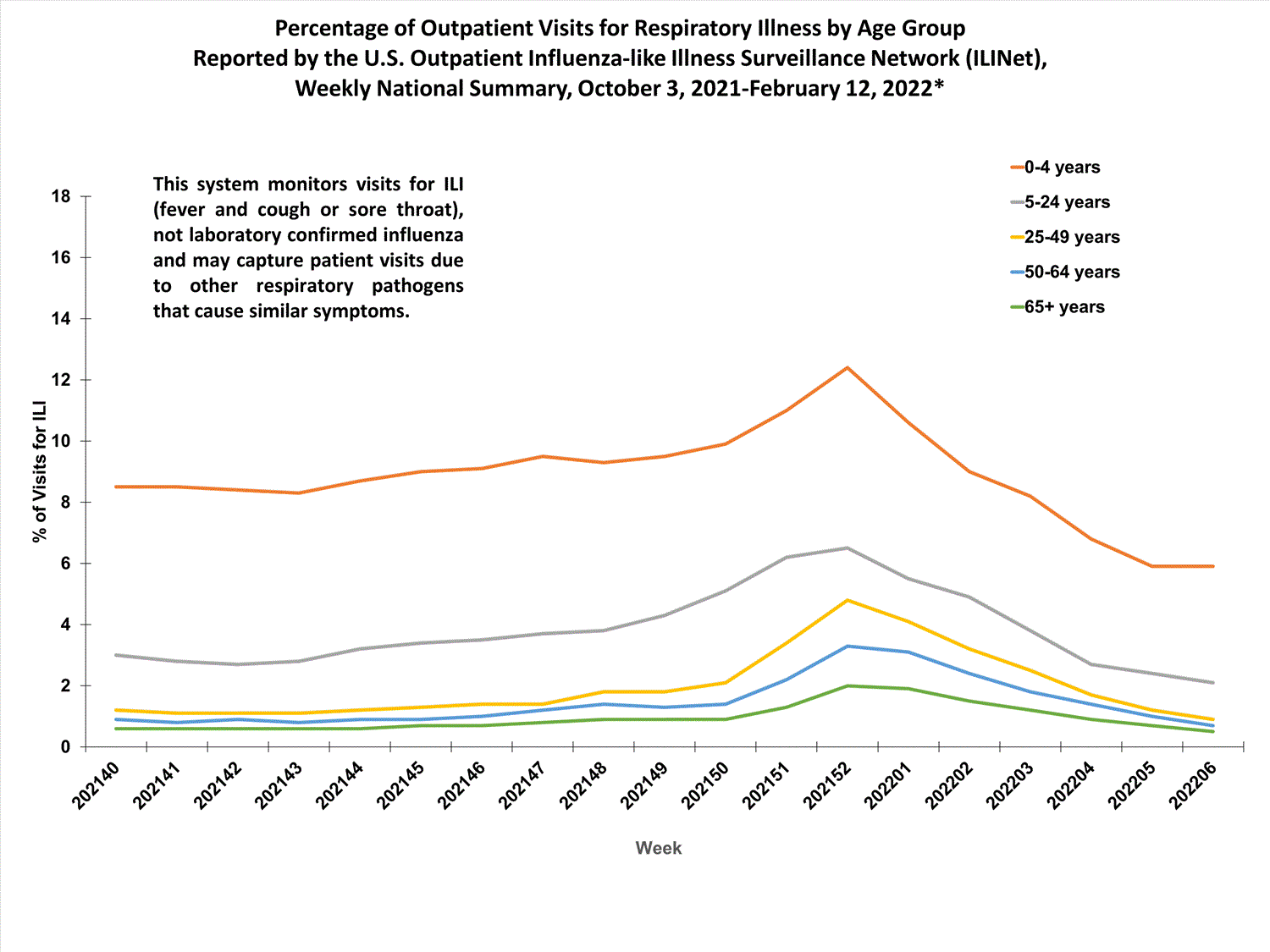
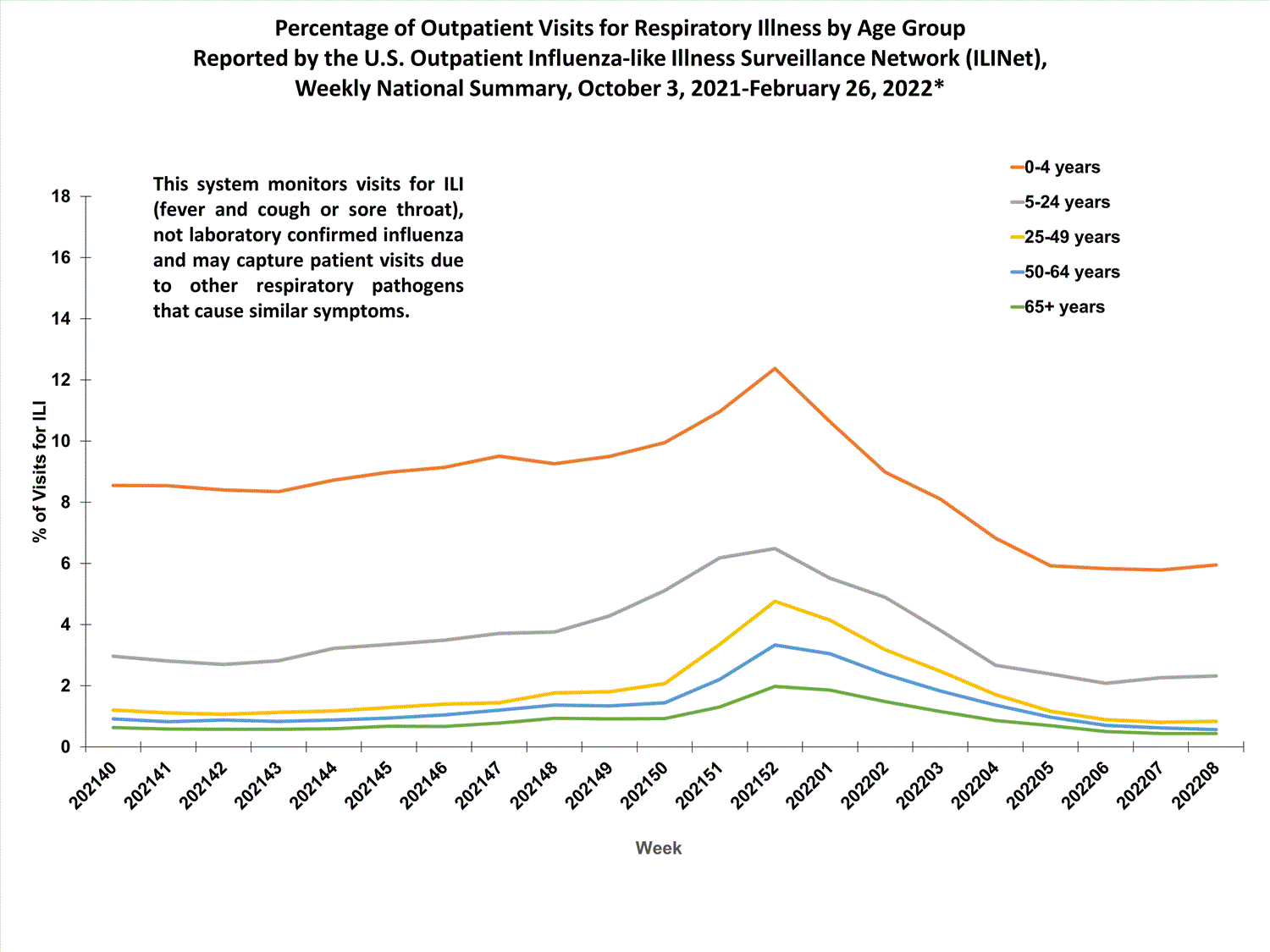
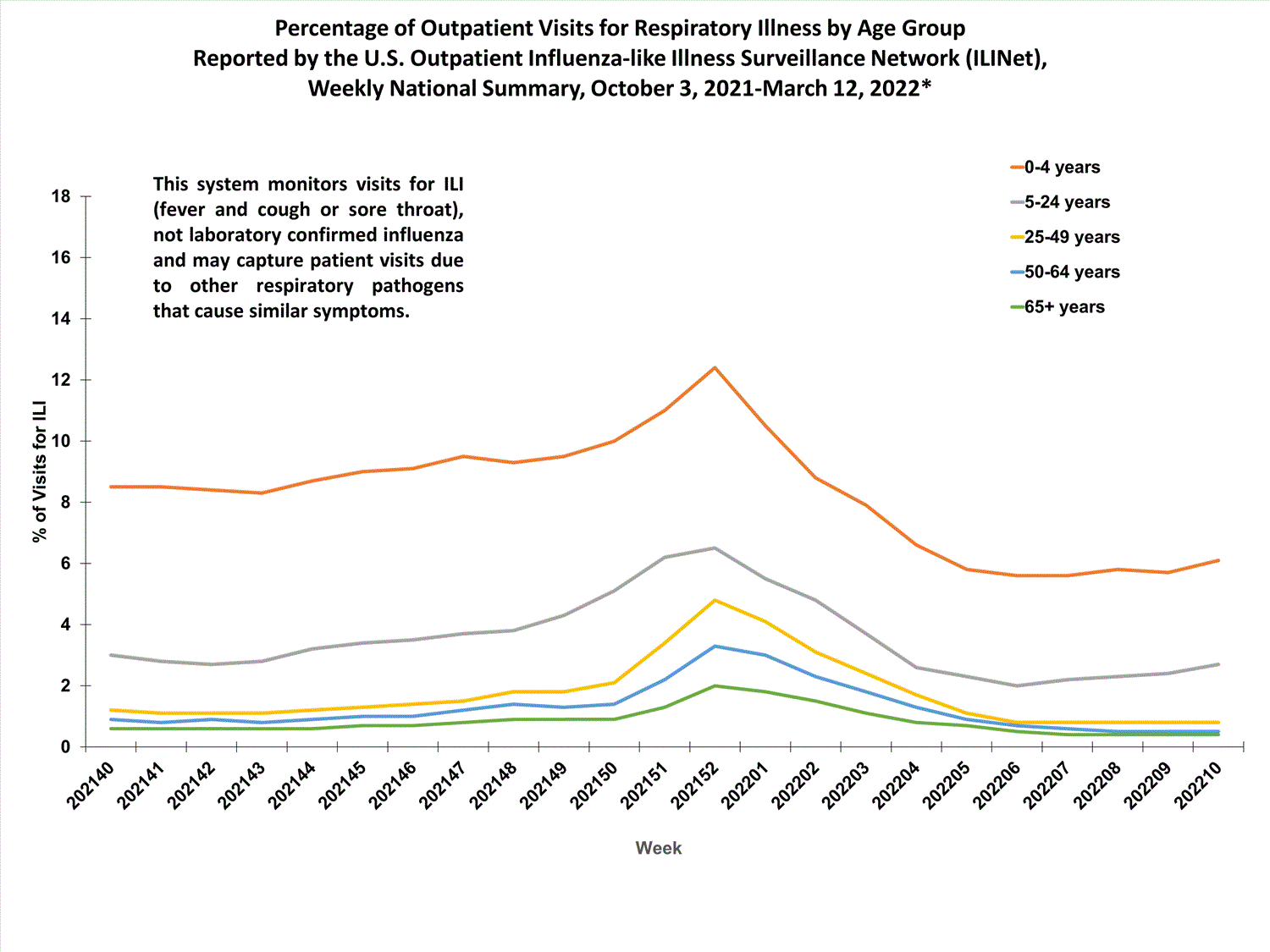
View Chart Data (current season only) | View Full ScreenOutpatient Respiratory Illness Visits by Age Group
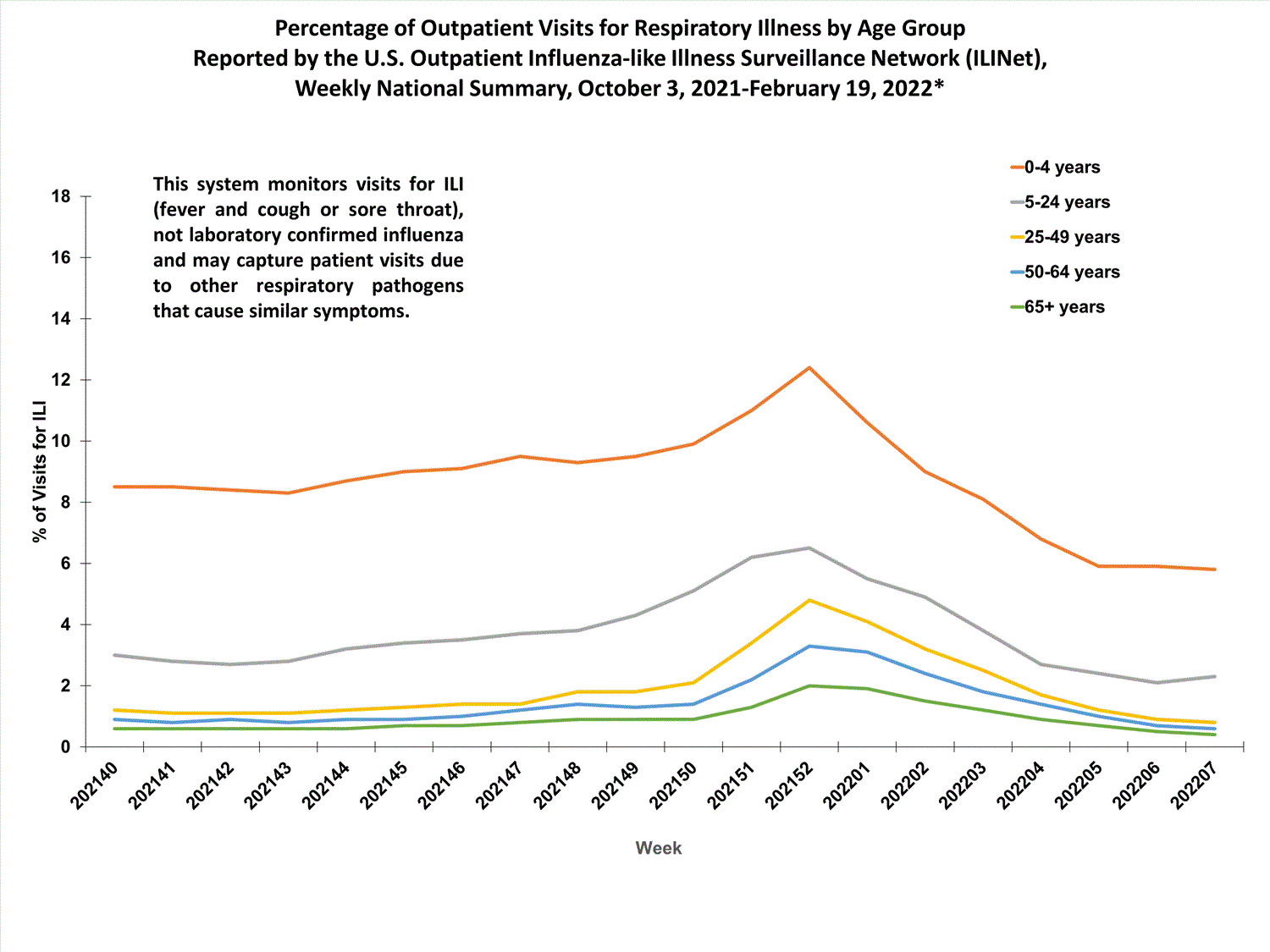
More than 70% of ILINet participants provide both the number of patient visits for respiratory illness and the total number of patient visits for the week broken out by age group. Data from this subset of providers are used to calculate the percentages of patient visits for respiratory illness by age group.
The percentage of visits for respiratory illness reported in ILINet increased for all age groups (0–4 years, 5–24 years, 25-49 years, 50–64 years, and 65+).
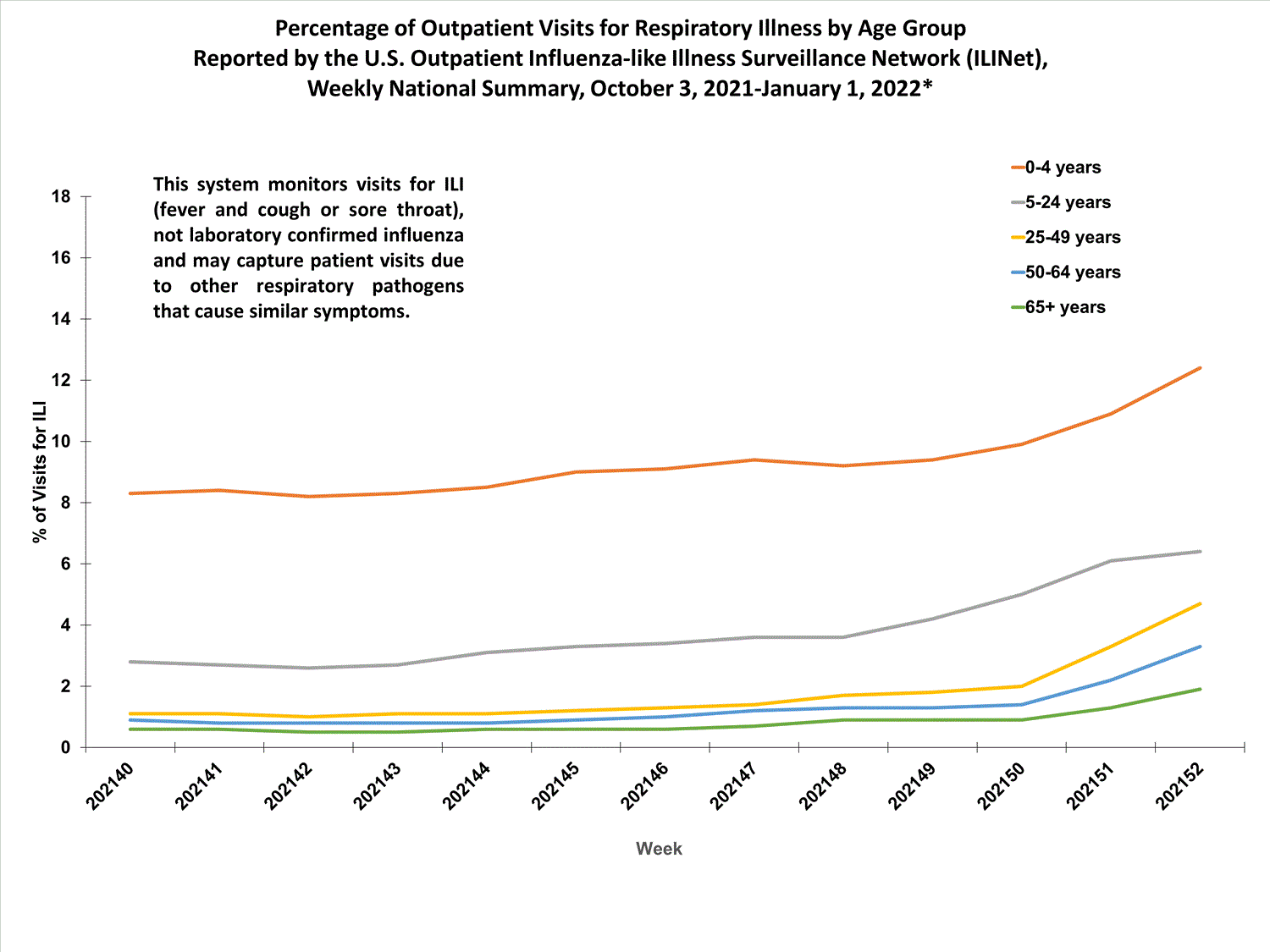
* Effective October 3, 2021 (week 40), the ILI definition (fever plus cough or sore throat) no longer includes “without a known cause other than influenza.”
View Chart Data | View Full Screen
Outpatient Respiratory Illness Activity Map
Data collected in ILINet are used to produce a measure of ILI activity* by state/jurisdiction and Core Based Statistical Areas (CBSA).
| Week 52 (Week ending Jan. 1, 2022) |
Week 51 (Week ending Dec. 25, 2021) |
Week 52 (Week ending Jan. 1, 2022) |
Week 51 (Week ending Dec. 25, 2021) |
|
| Very High | 9 | 3 | 32 | 7 |
| High | 22 | 17 | 134 | 81 |
| Moderate | 10 | 14 | 107 | 110 |
| Low | 4 | 9 | 143 | 163 |
| Minimal | 9 | 11 | 223 | 295 |
| Insufficient Data | 1 | 1 | 290 | 273 |
*Data collected in ILINet may disproportionally represent certain populations within a jurisdiction or CBSA, and therefore, may not accurately depict the full picture of influenza activity for the entire jurisdiction or CBSA. Differences in the data presented here by CDC and independently by some health departments likely represent differing levels of data completeness with data presented by the health department likely being the more complete.
Additional information about medically attended visits for ILI for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or ILI Activity Map
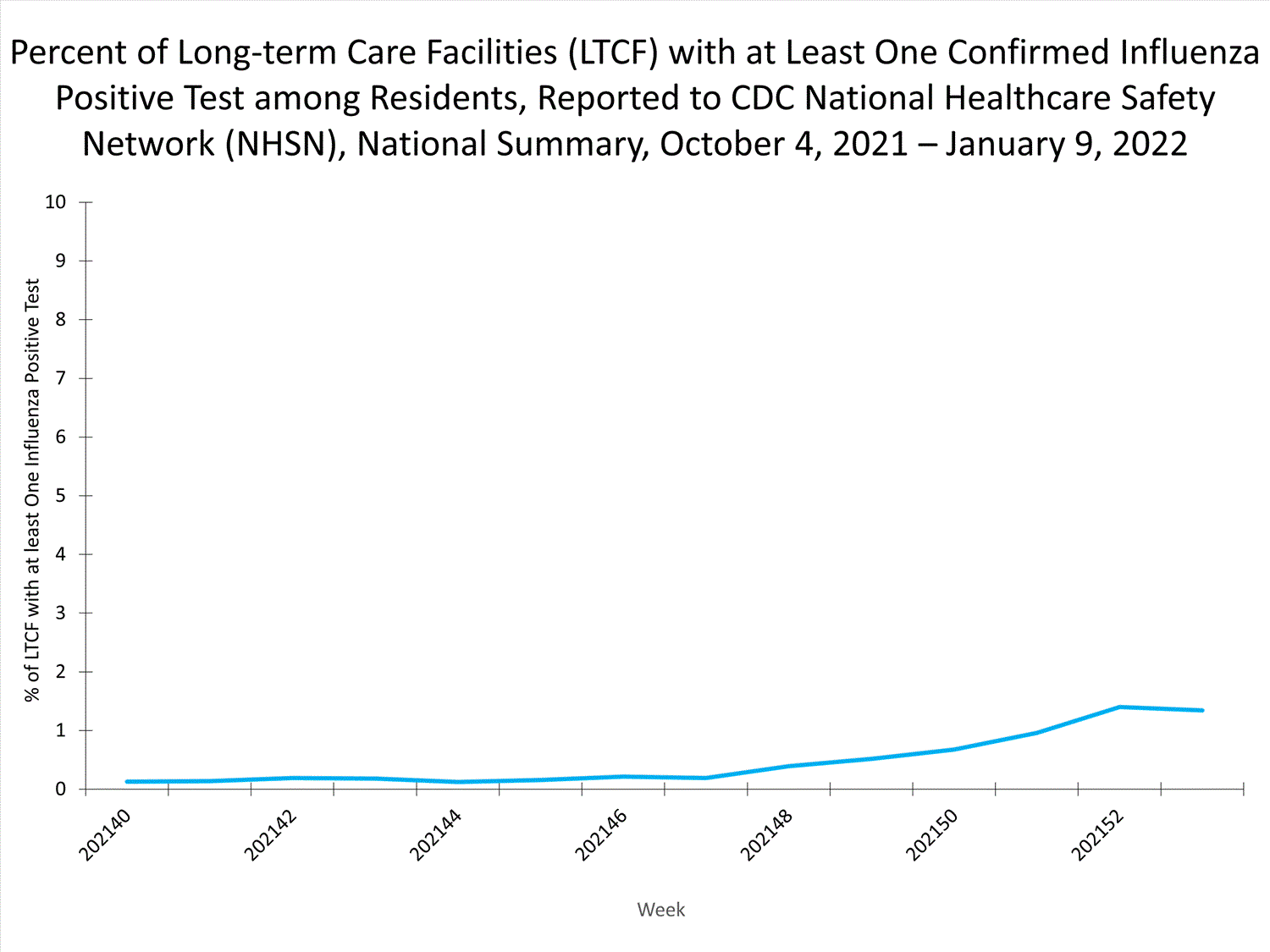
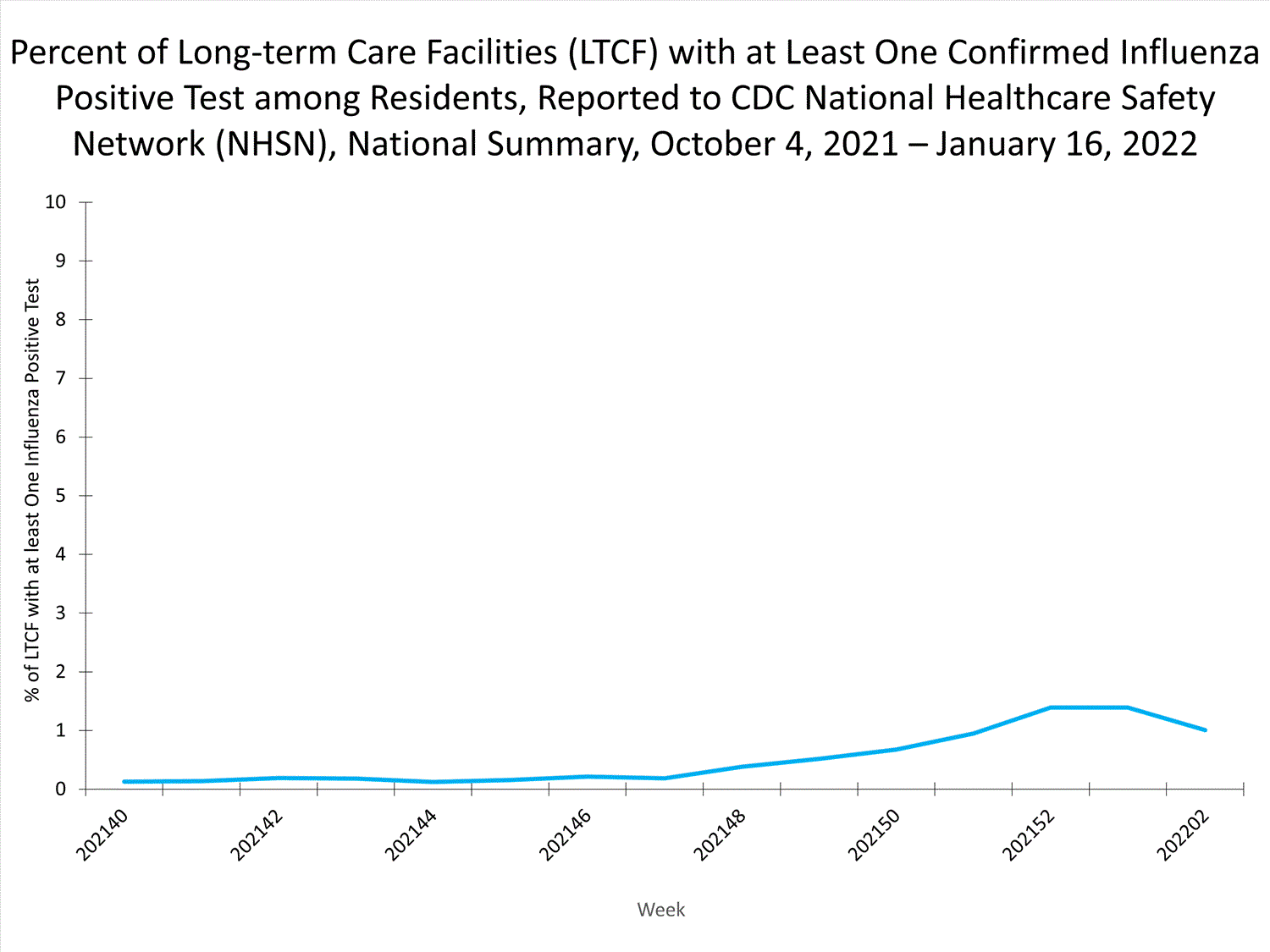
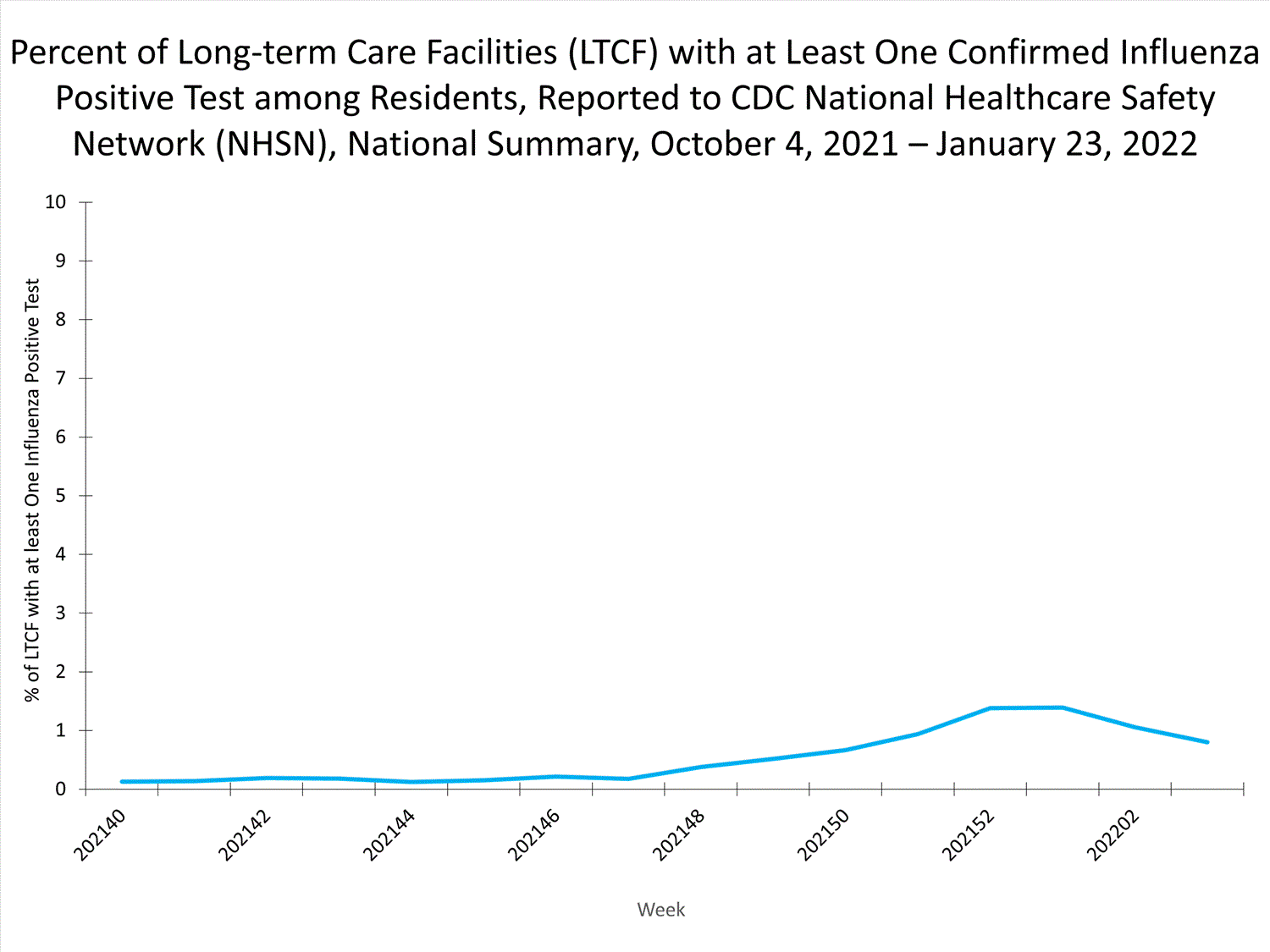
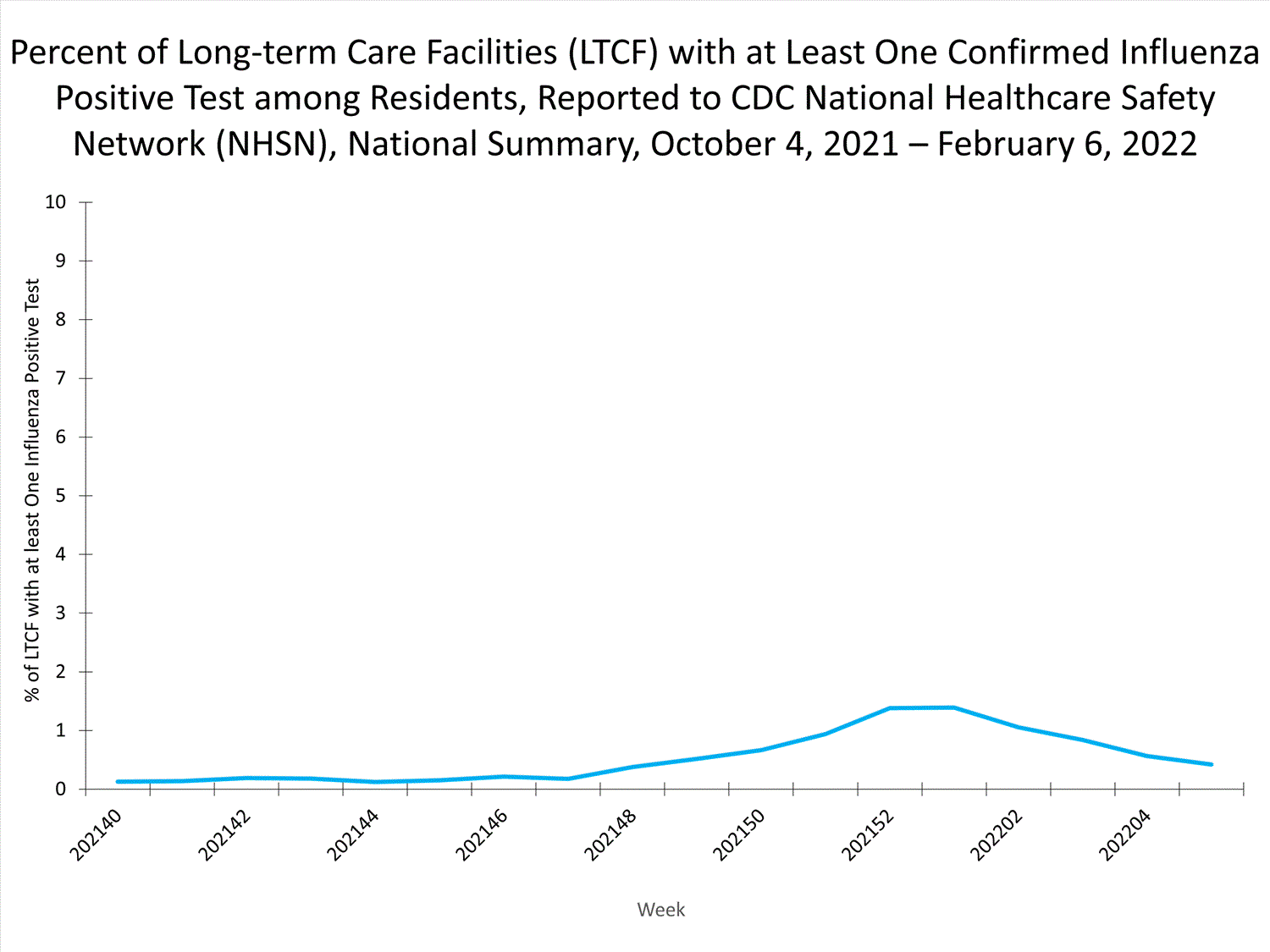
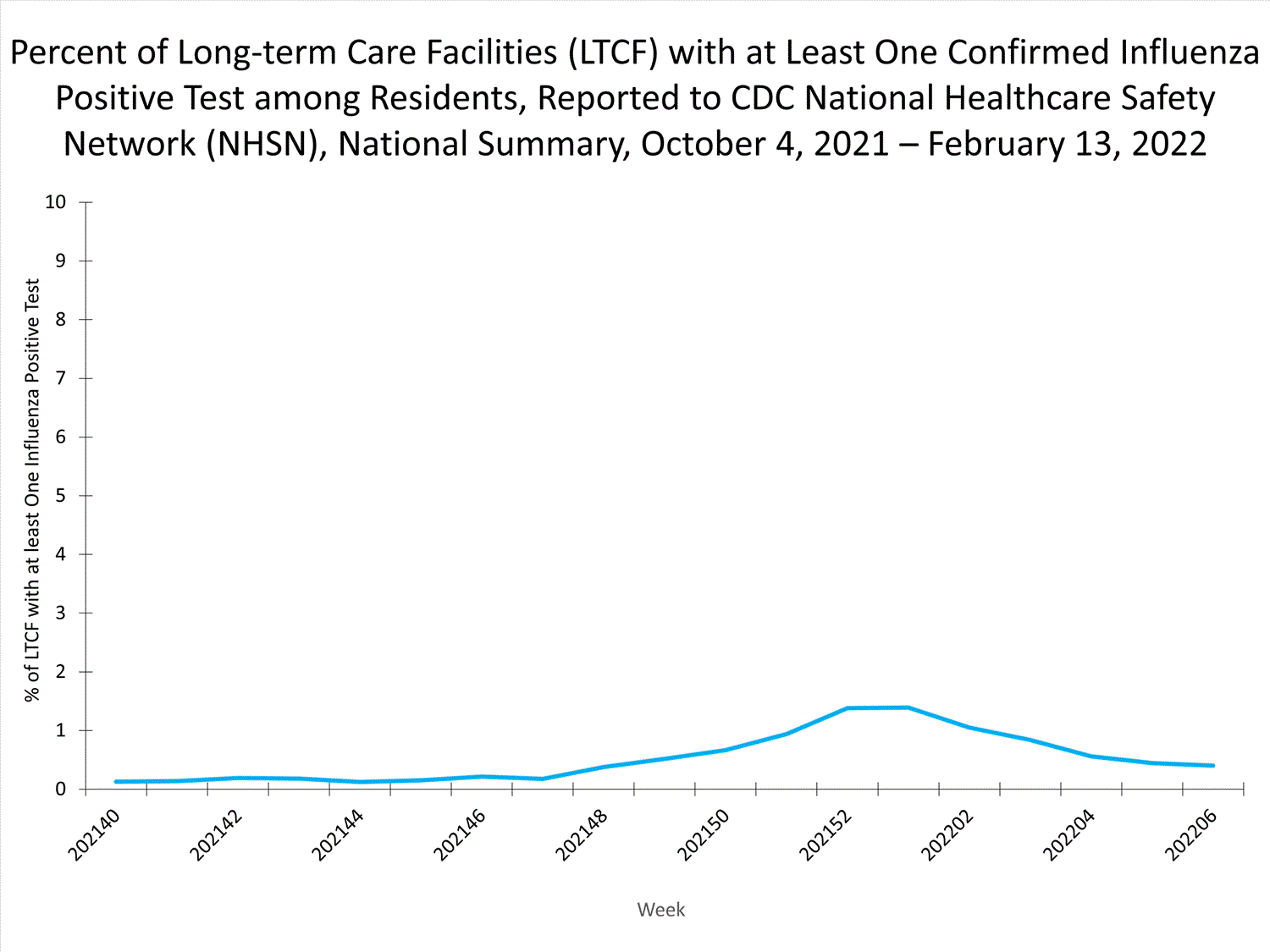
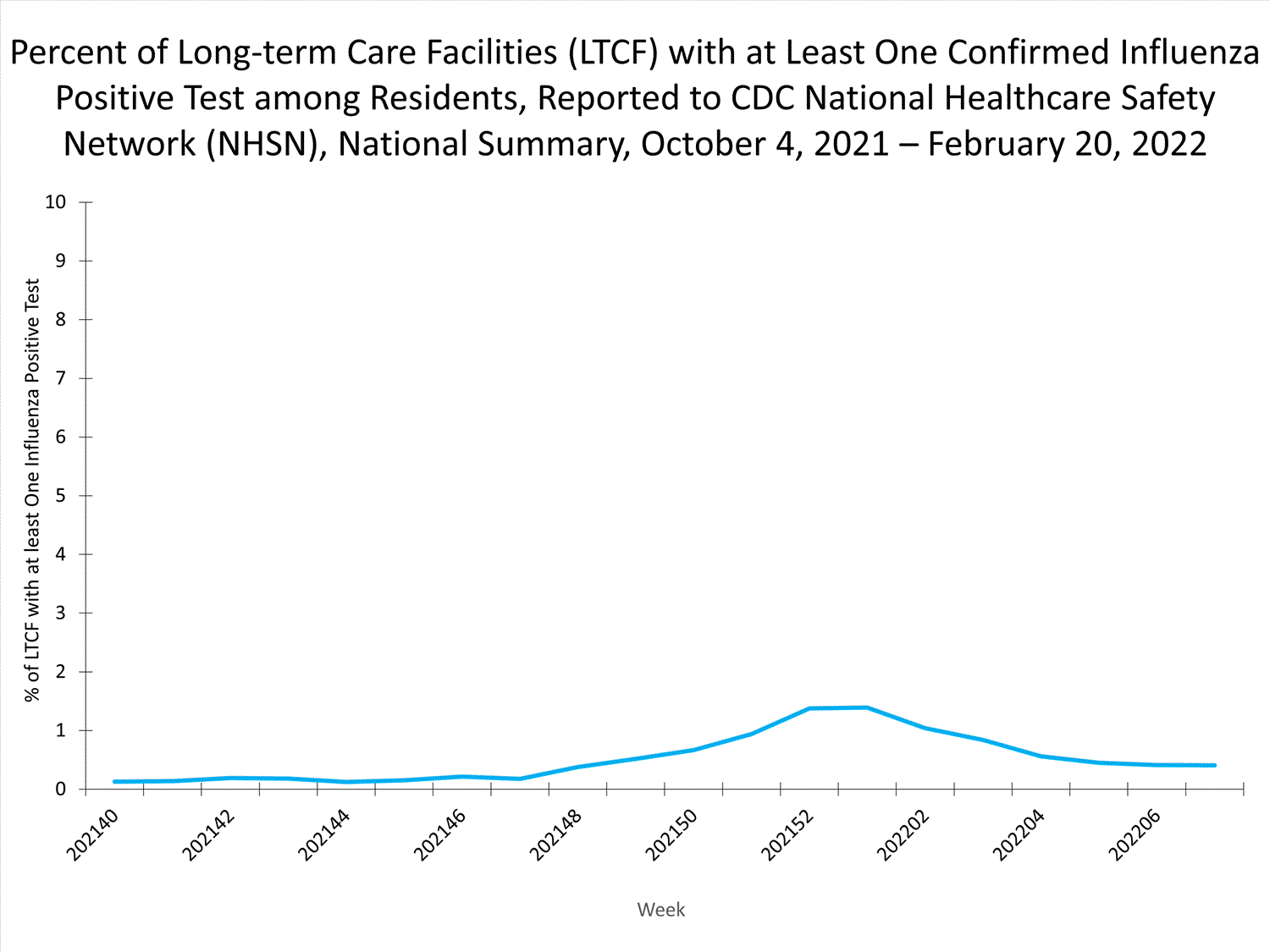
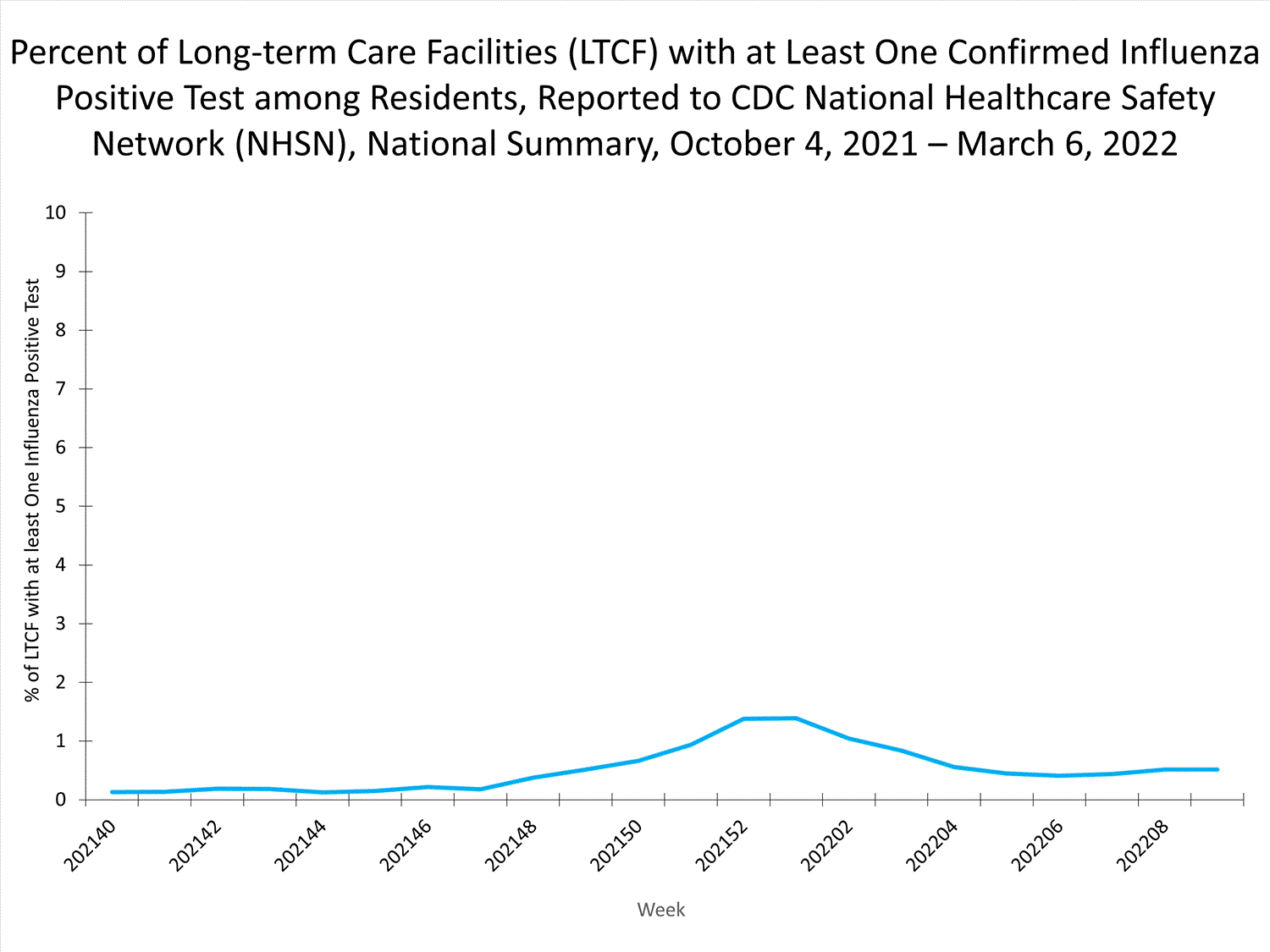
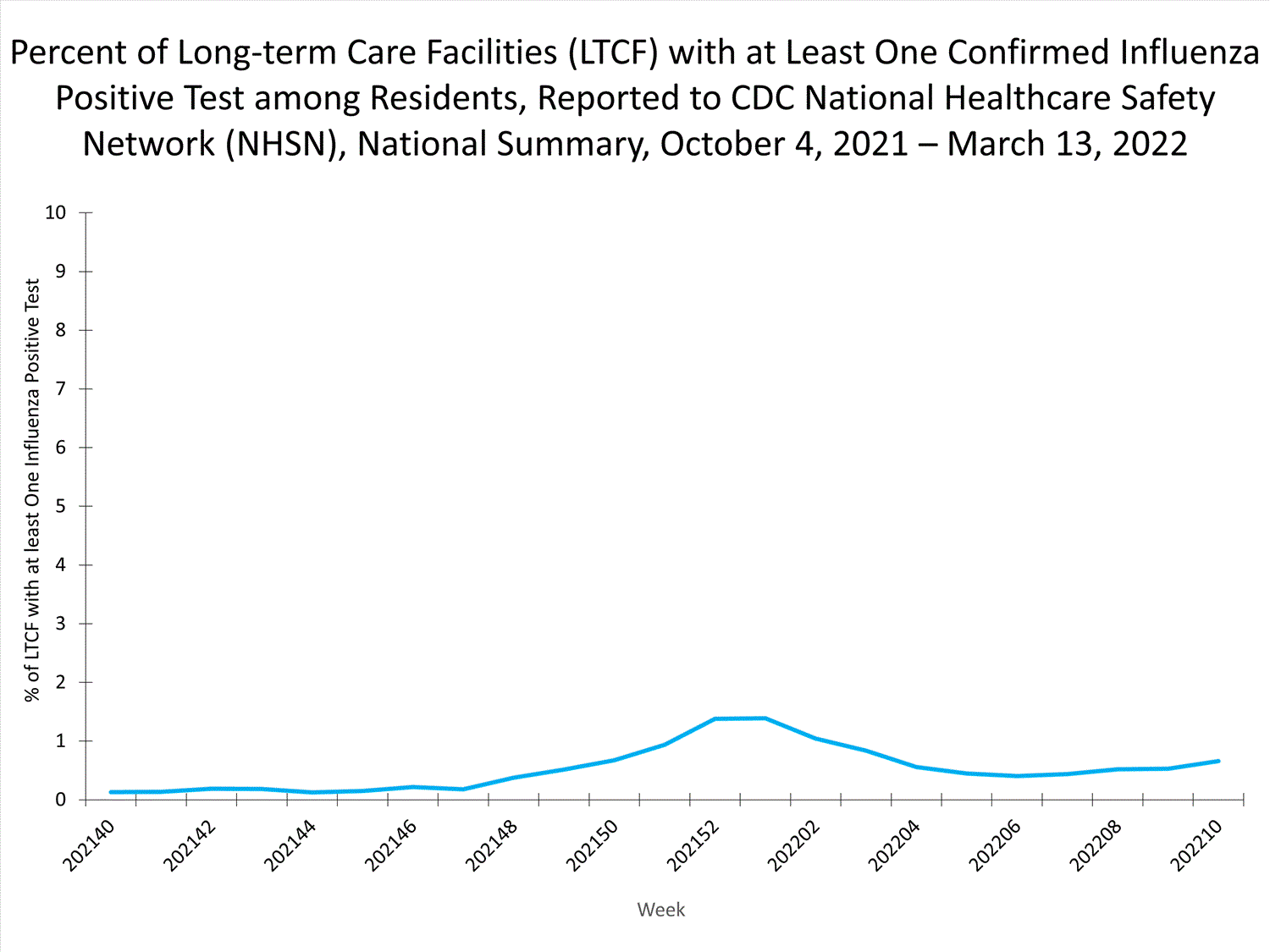
Long-term Care Facility (LTCF) Surveillance
LTCFs (e.g., nursing homes/skilled nursing, long-term care for the developmentally disabled, and assisted living facilities) from all 50 states and U.S. territories report data on influenza infections among residents through the National Healthcare Safety Network (NHSN) Long-term Care Facility Component. During week 52, 185 (1.3%) of 14,141 reporting LTCFs reported at least one influenza positive test among their residents.
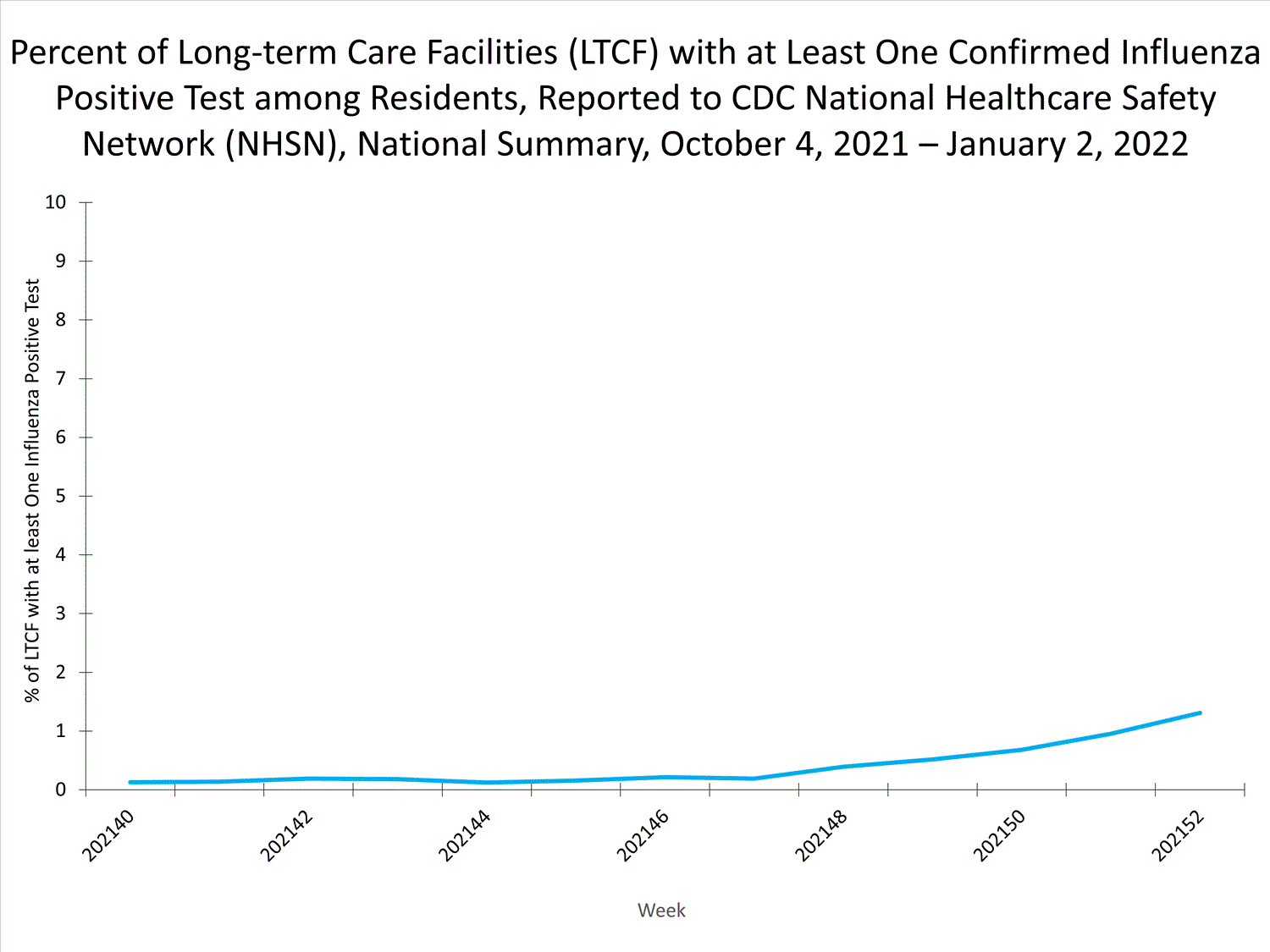
View Chart Dataexcel icon | View Full Screen
Additional information about long-term care facility surveillance:
Surveillance Methods | Additional Dataexternal icon
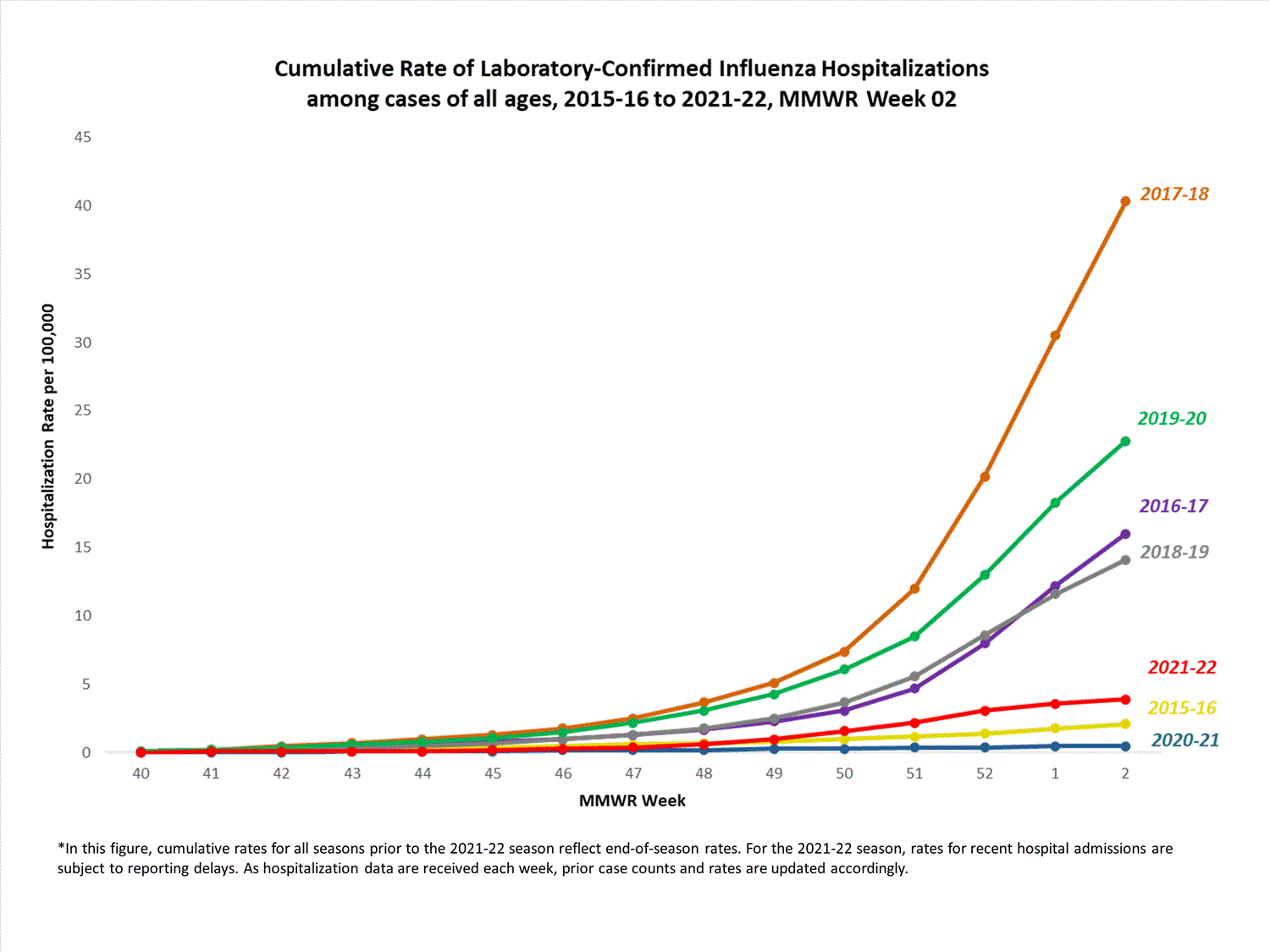
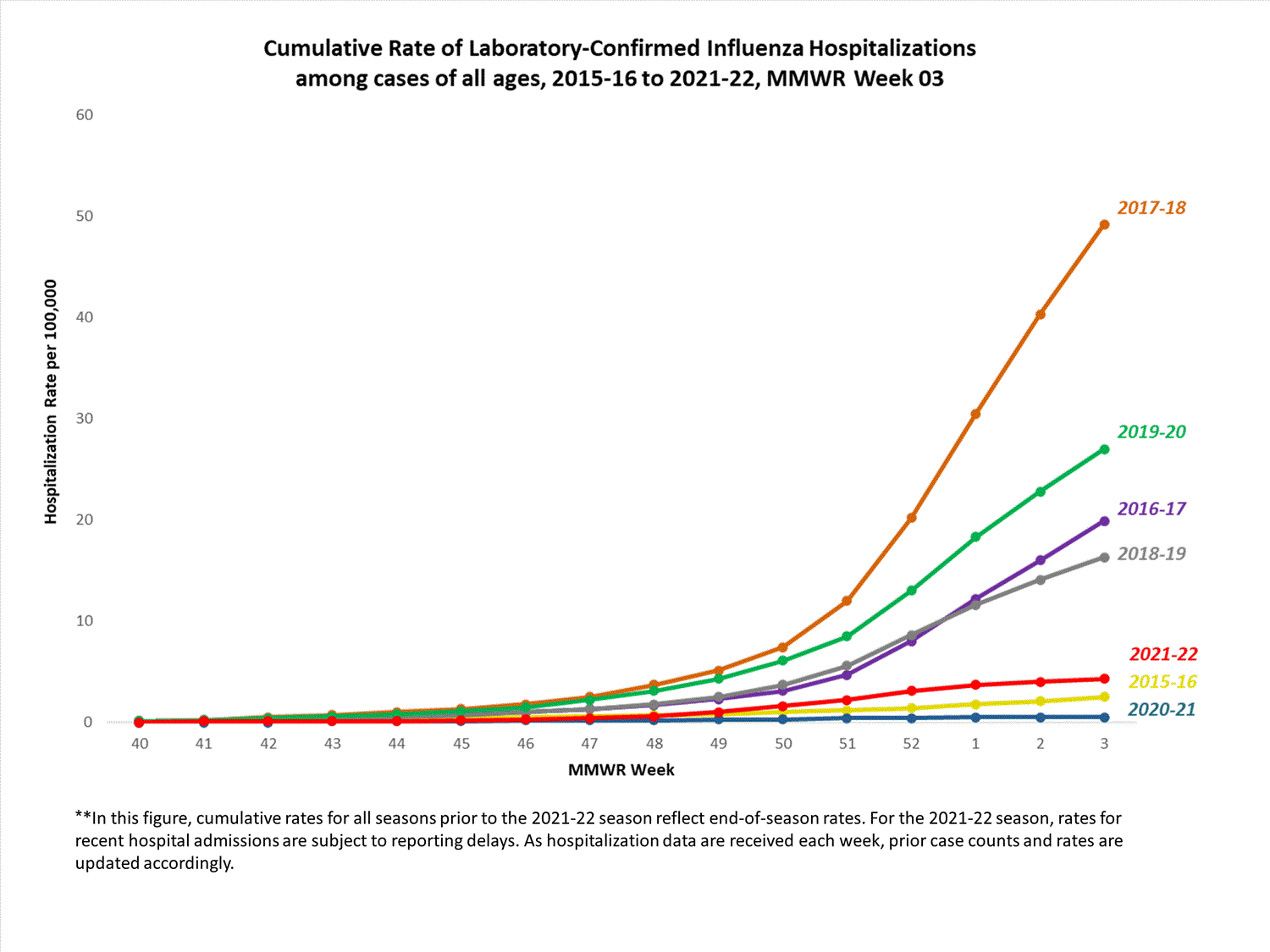
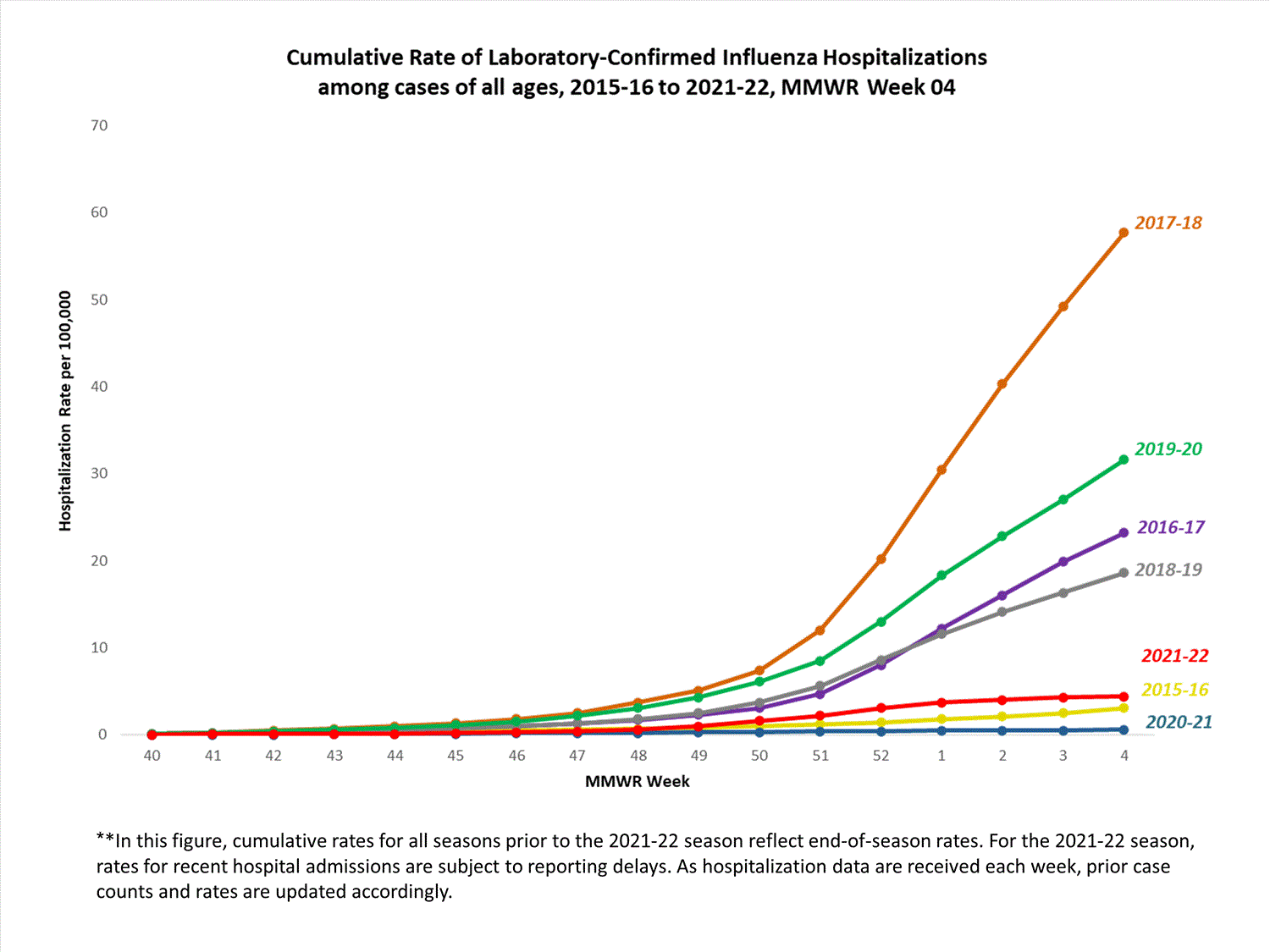
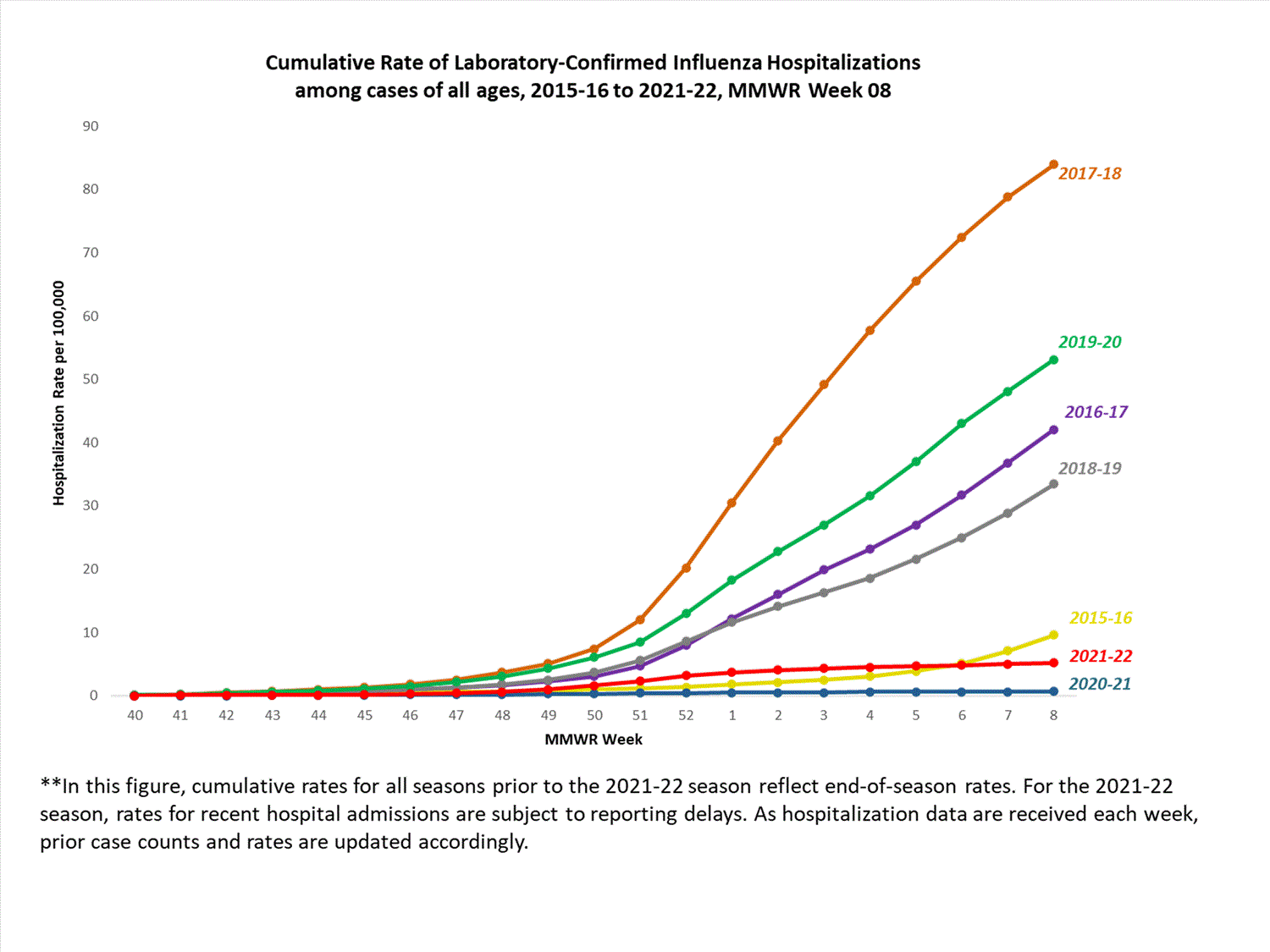
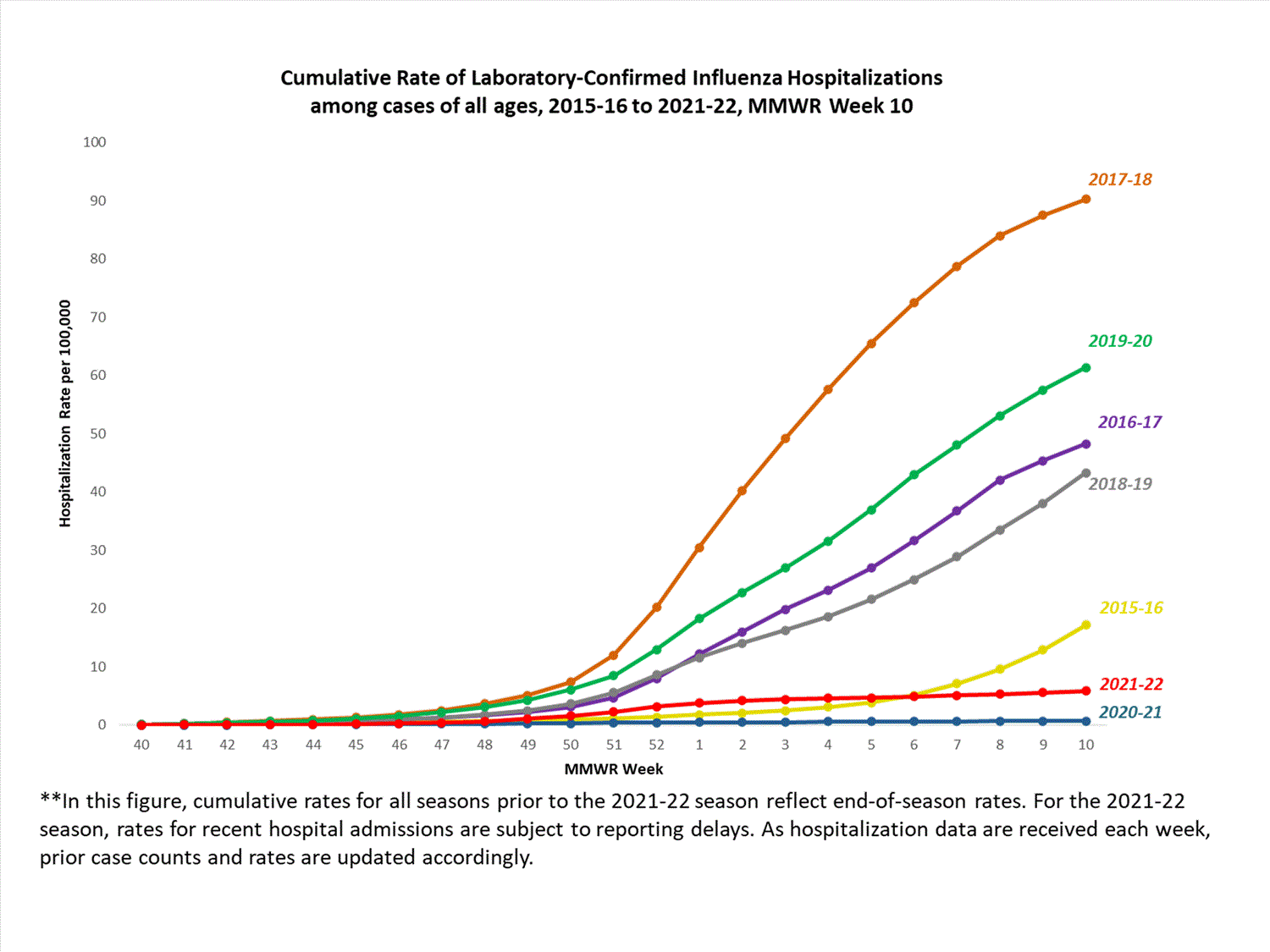
Hospitalization Surveillance
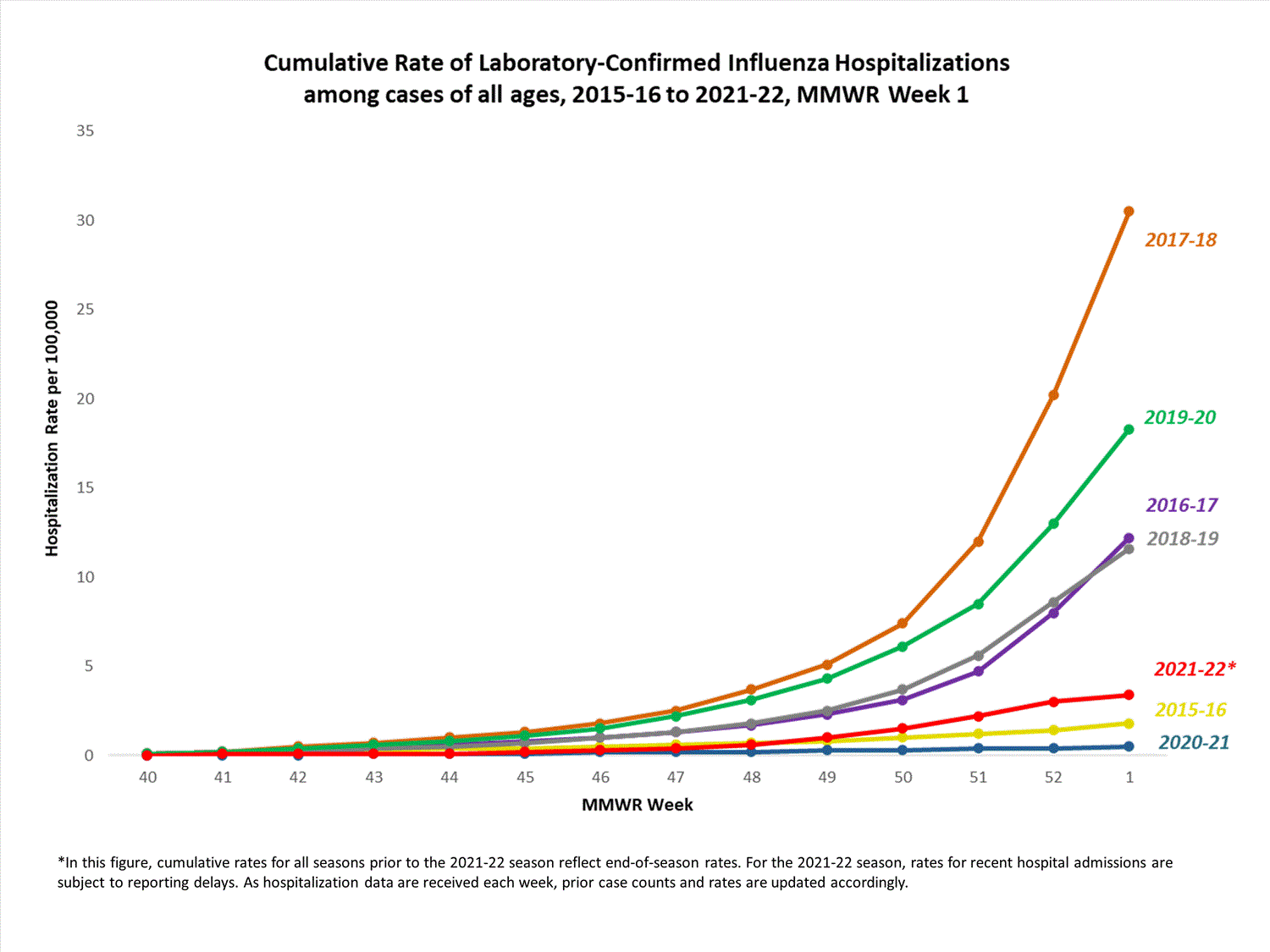
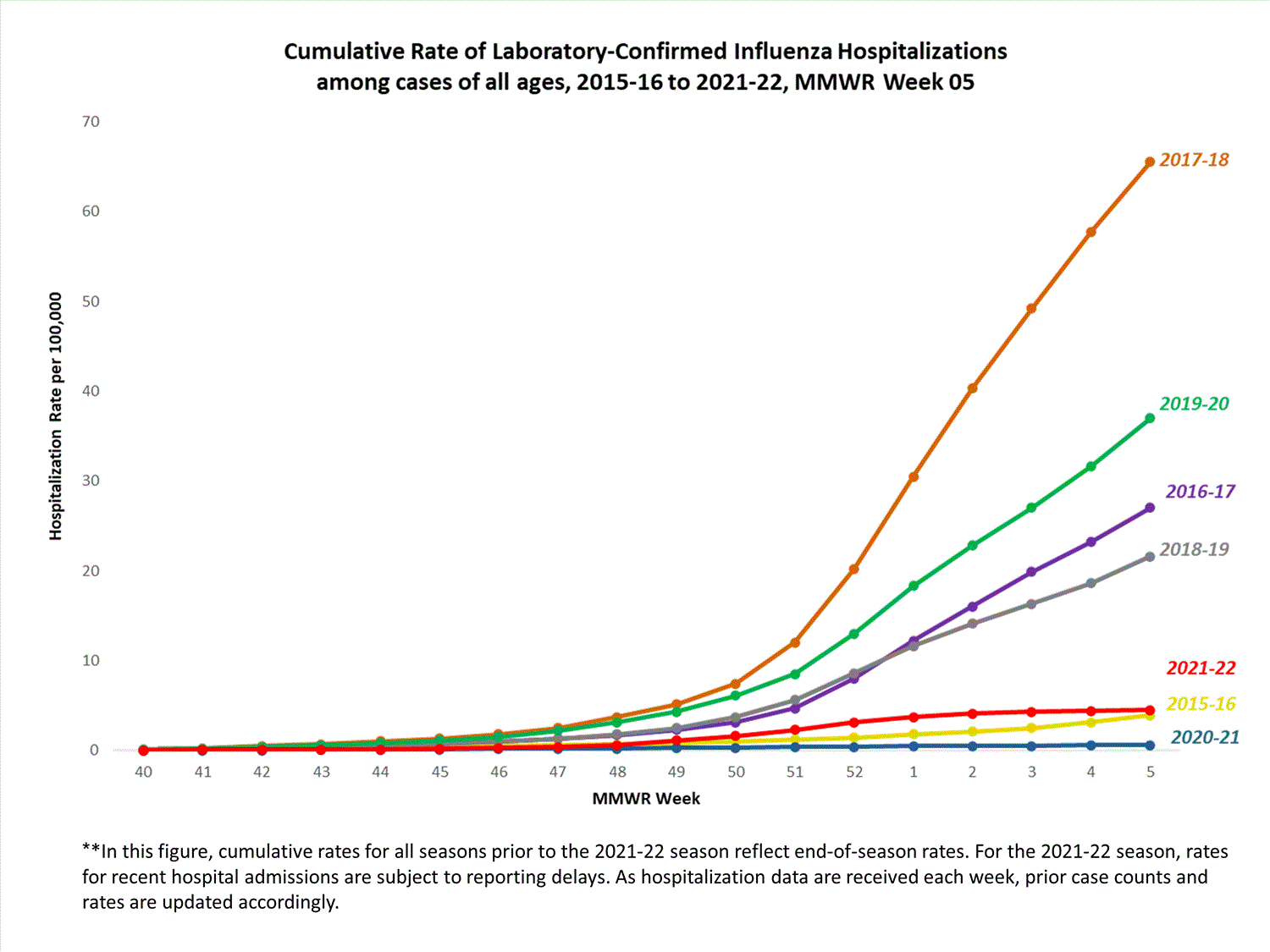
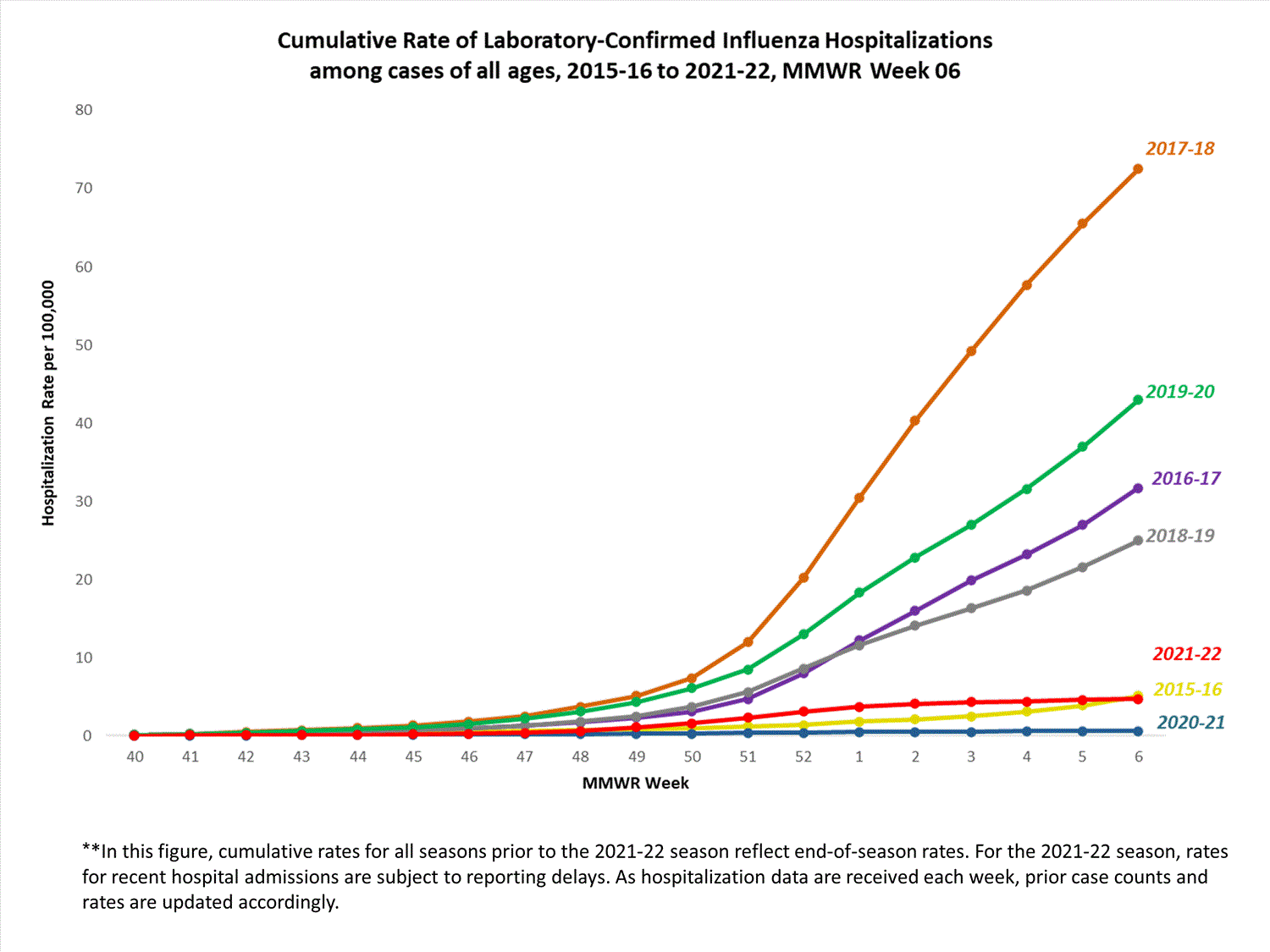
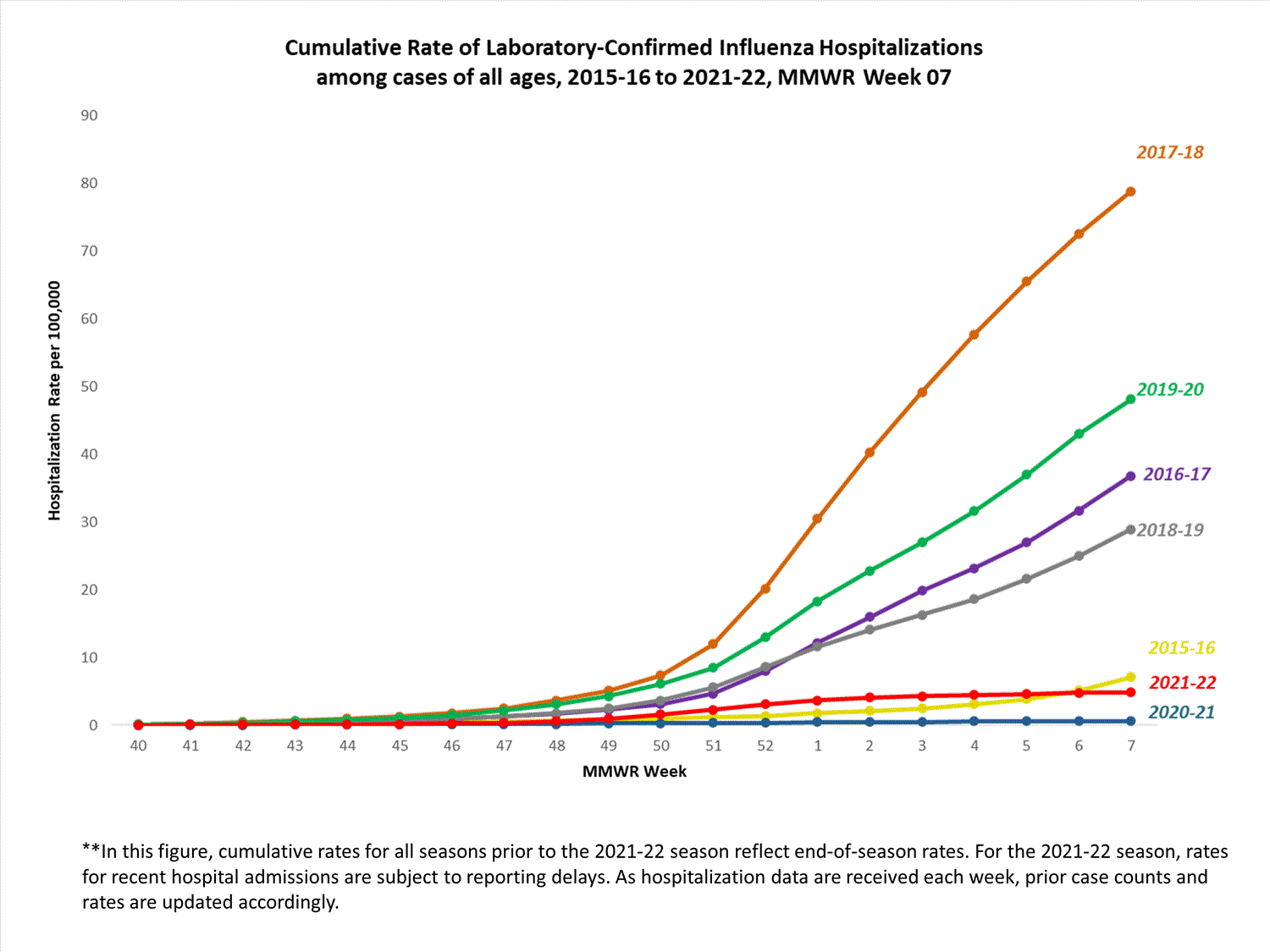
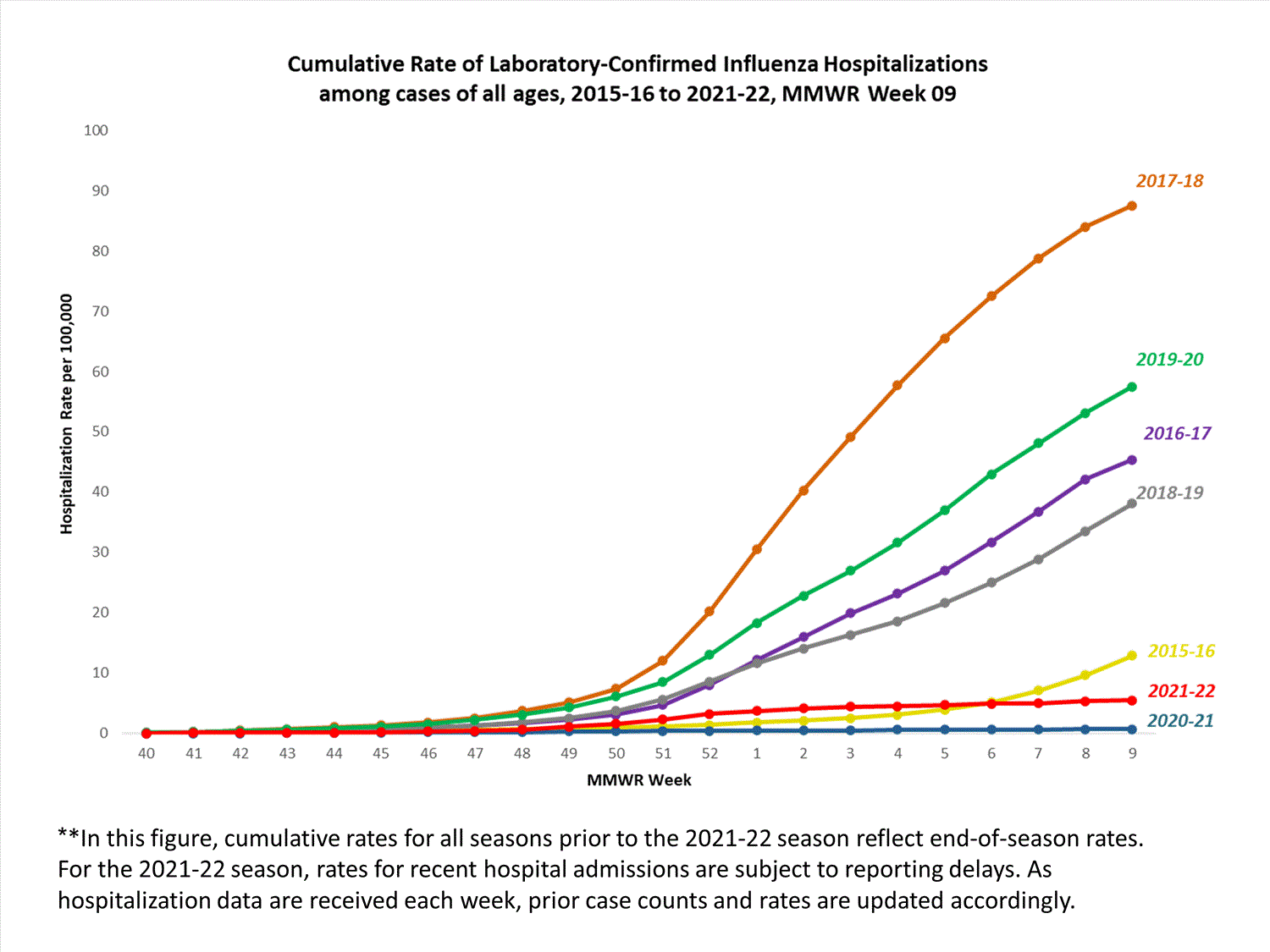
FluSurv-NET
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in 14 states and represents approximately 9% of the U.S. population. FluSurv-NET hospitalization data are preliminary. Case counts and rates for recent hospital admissions are subject to reporting delays; these delays are likely to be more pronounced around holidays. As hospitalization data are received each week, prior case counts and rates are updated accordingly. As such, end-of-season rates for any given week may vary substantially from in-season reported rates.
A total of 761 laboratory-confirmed influenza-associated hospitalizations were reported by FluSurv-NET sites between October 1, 2021, and January 1, 2022. The overall cumulative hospitalization rate was 2.6 per 100,000 population. This cumulative hospitalization rate is higher than the cumulative in-season hospitalization rate observed in week 52 during the 2020-2021 season (0.3 per 100,000), but lower than the in-season rates observed in week 52 during the 4 seasons preceding the COVID-19 pandemic (ranged from 4.9 to 13.8 per 100,000 during the 2016-17 through 2019-20 seasons). The highest rate of hospitalization was among adults aged ≥65 (7.2 per 100,000 population), followed by children aged 0-4 (3.2 per 100,000 population) and adults aged 50-64 (2.2 per 100,000 population). Among 761 hospitalizations, 715 (94.0%) were associated with influenza A virus, 42 (5.5%) with influenza B virus, 2 (0.3%) with influenza A virus and influenza B virus co-infection, and 2 (0.3%) with influenza virus for which the type was not determined. Among 166 hospitalizations with influenza A subtype information, 166 (100%) were A(H3N2).
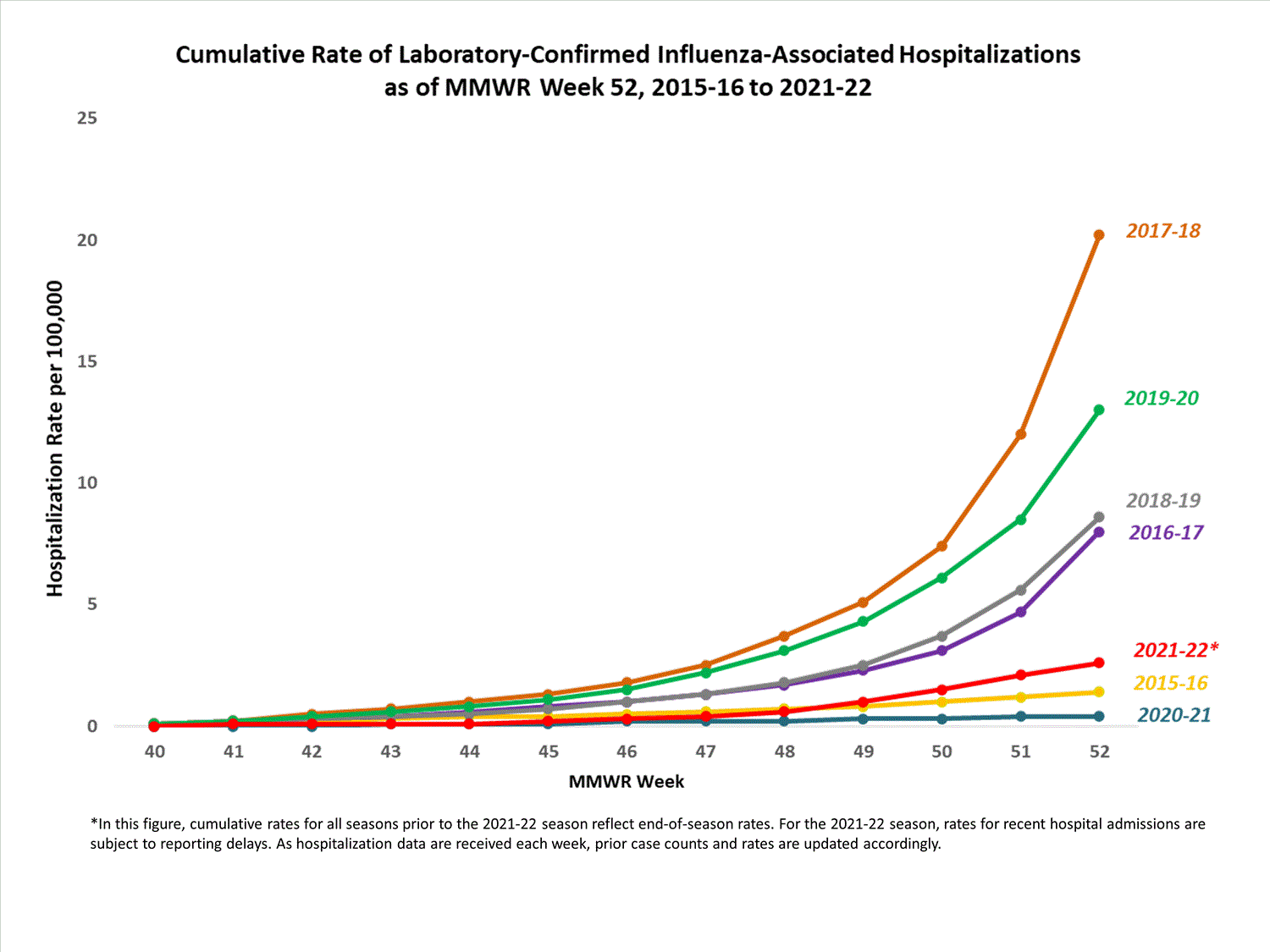
View Full Screen
Additional FluSurv-NET hospitalization surveillance information for current and past seasons and additional age groups:
Surveillance Methods | FluView Interactive
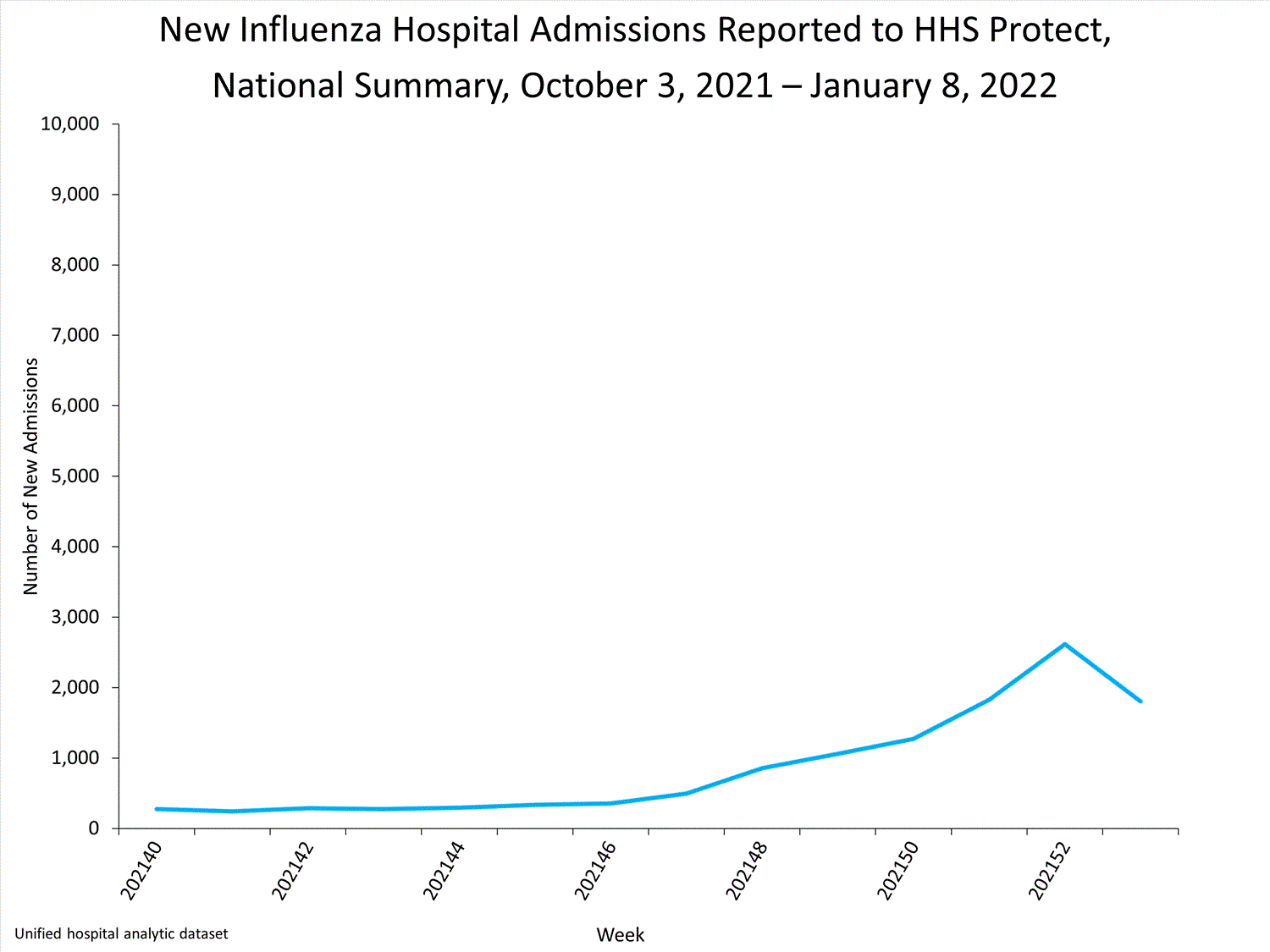
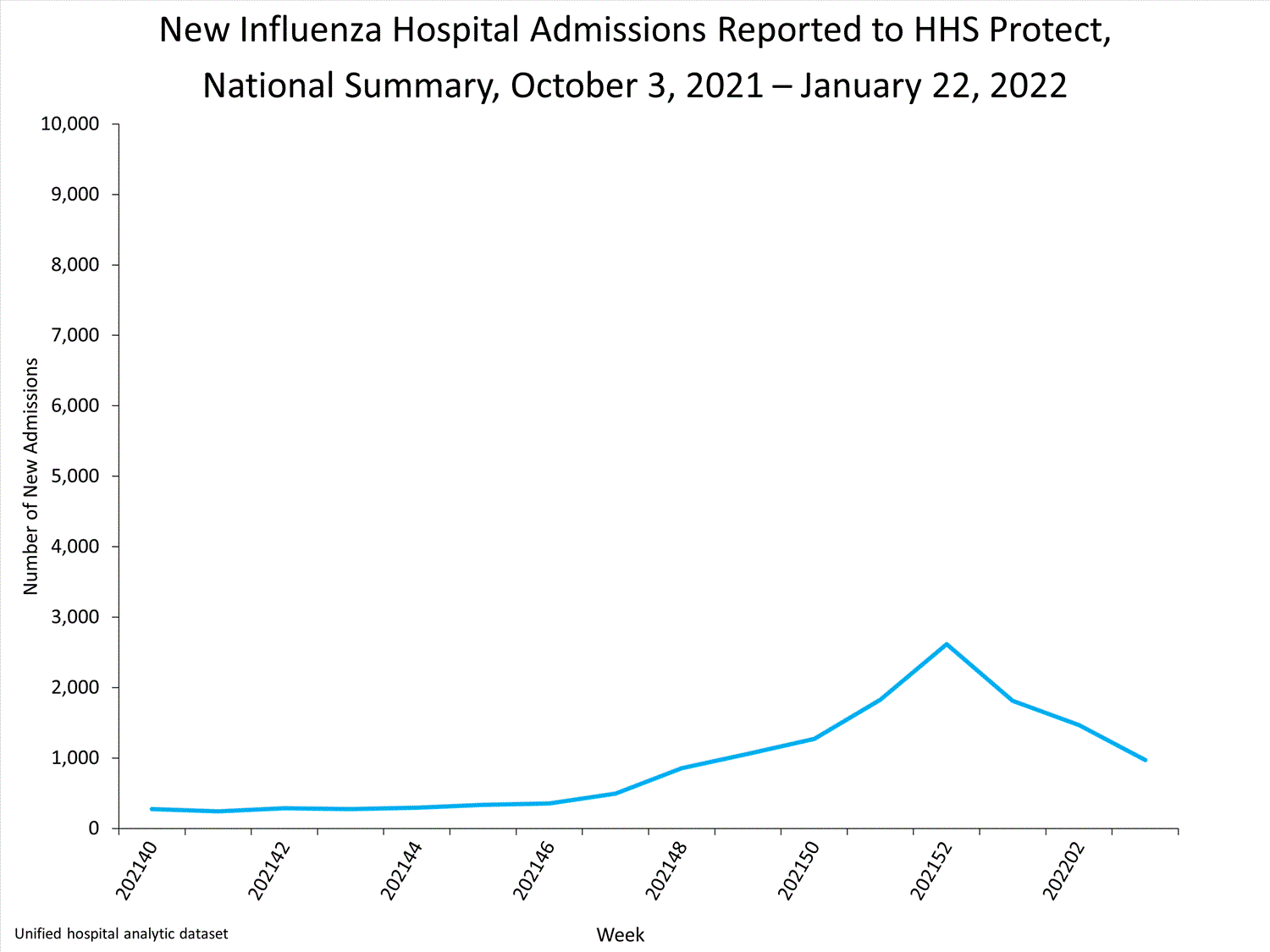
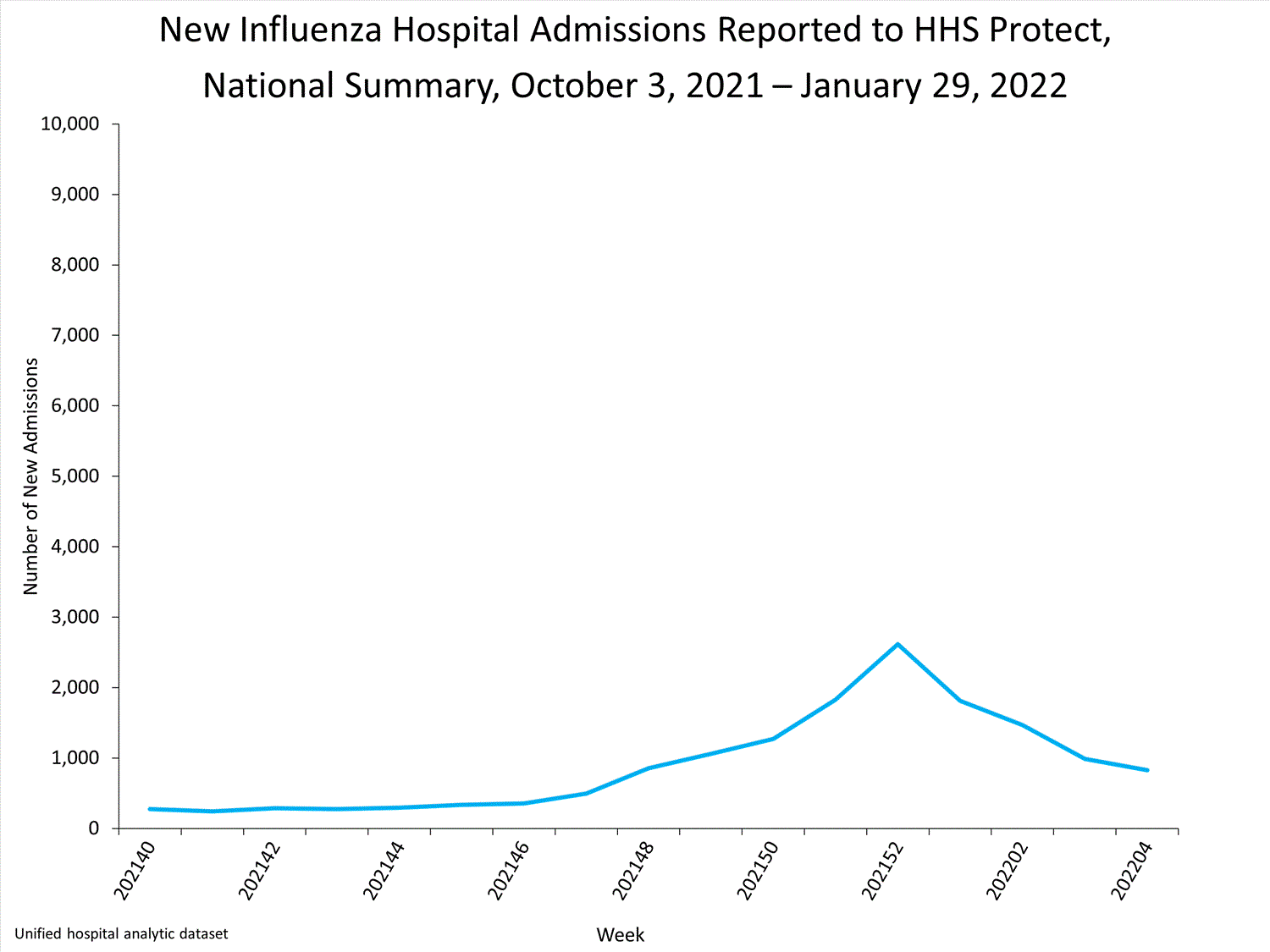
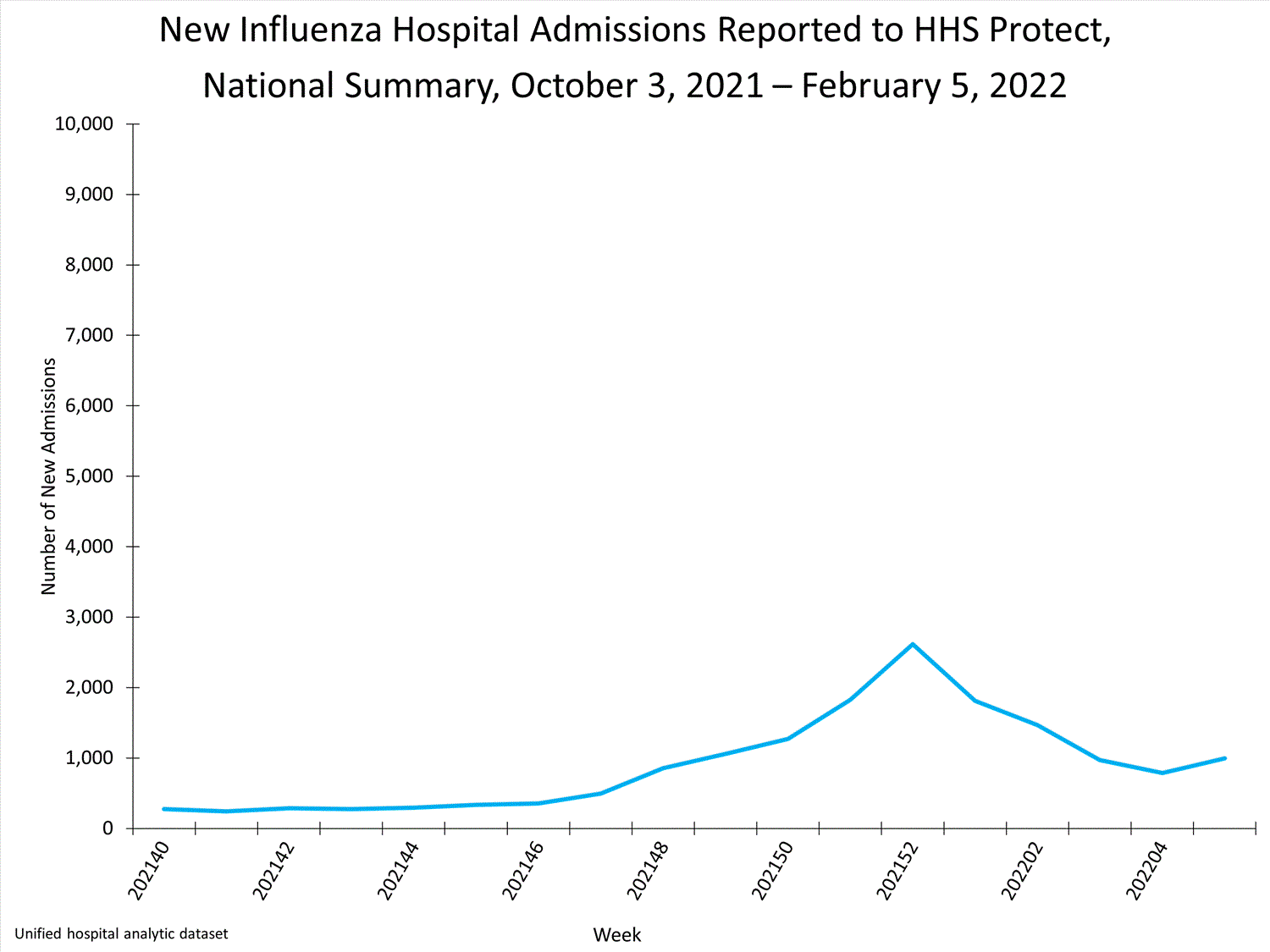
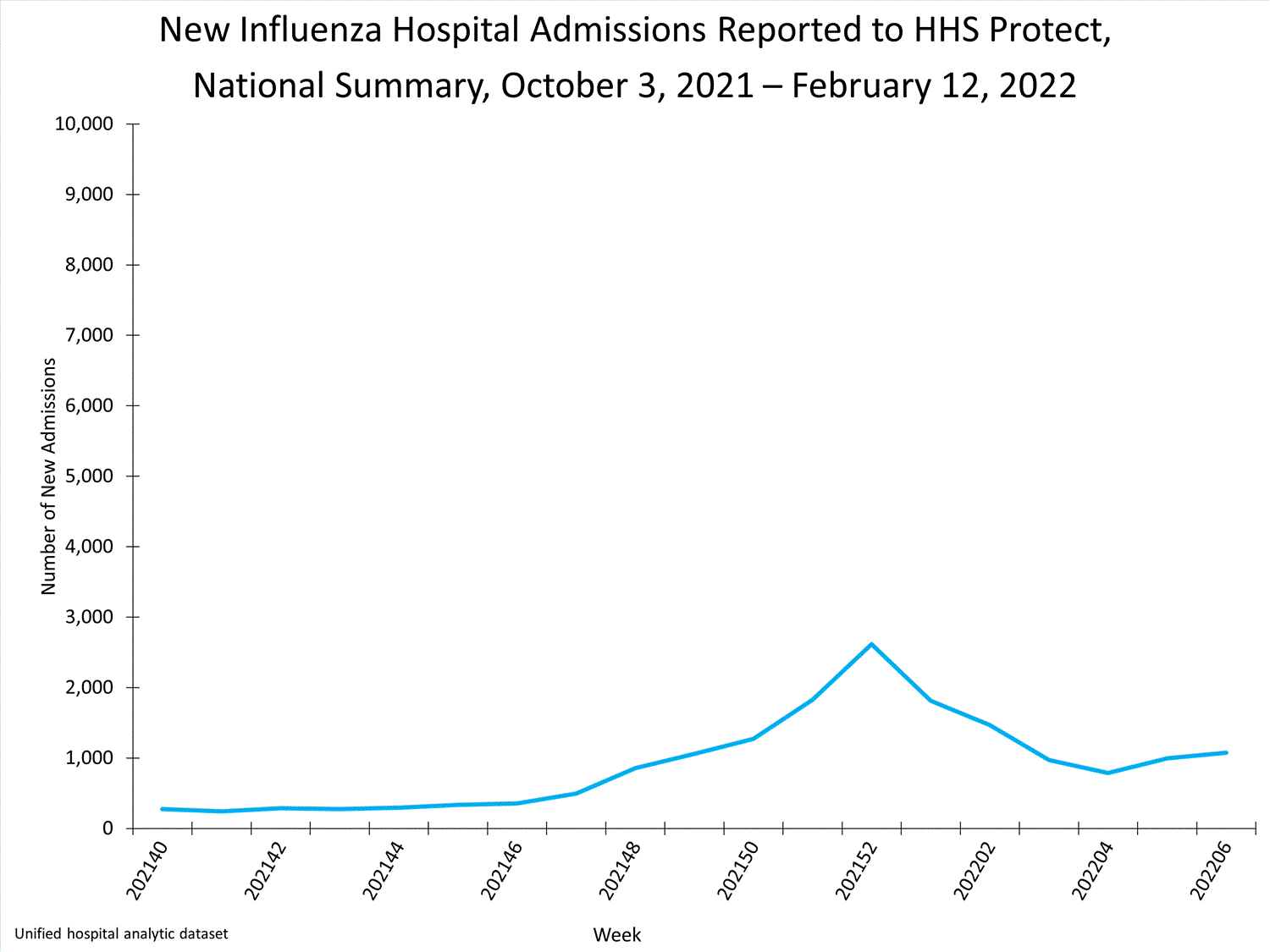
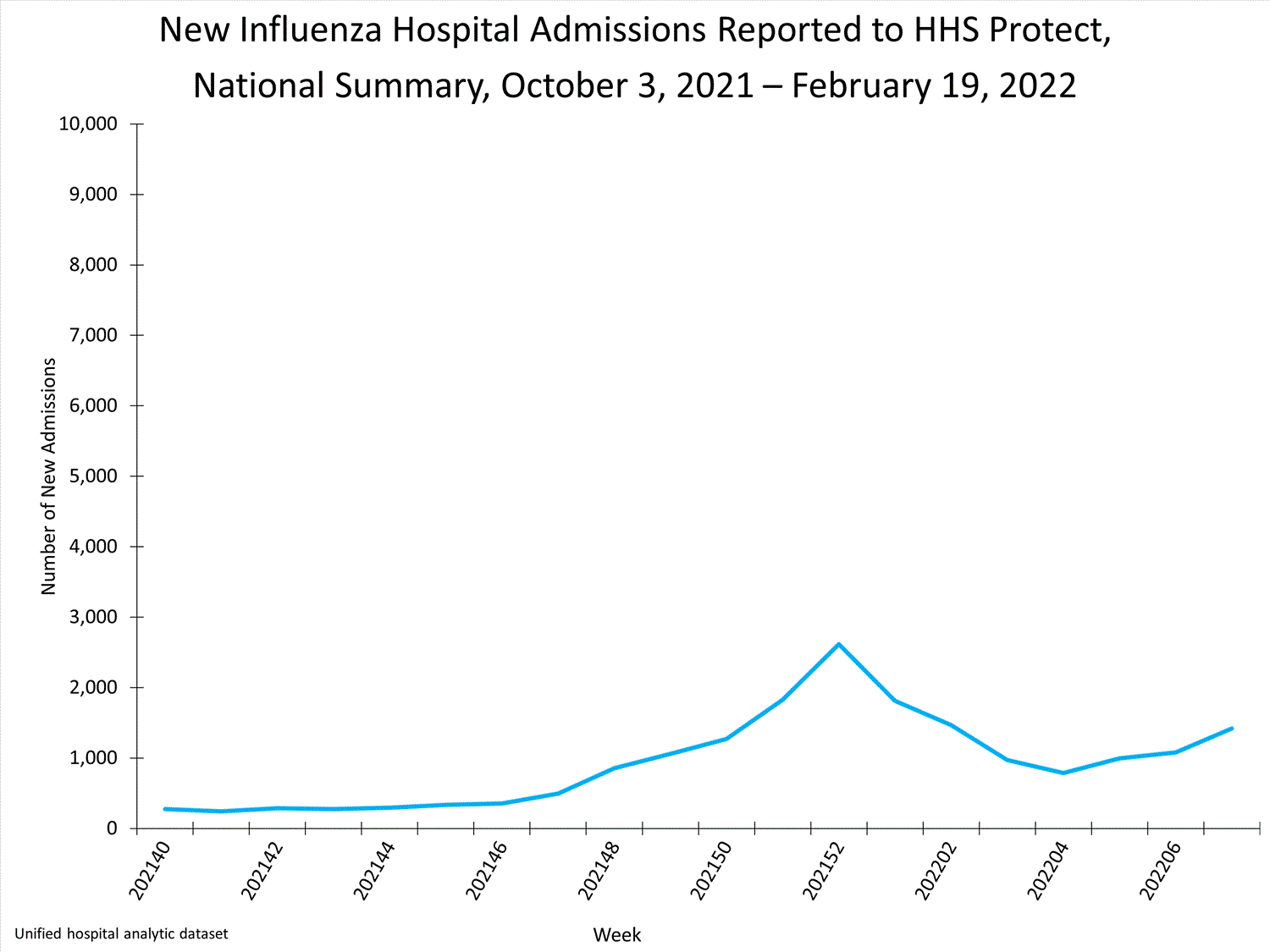
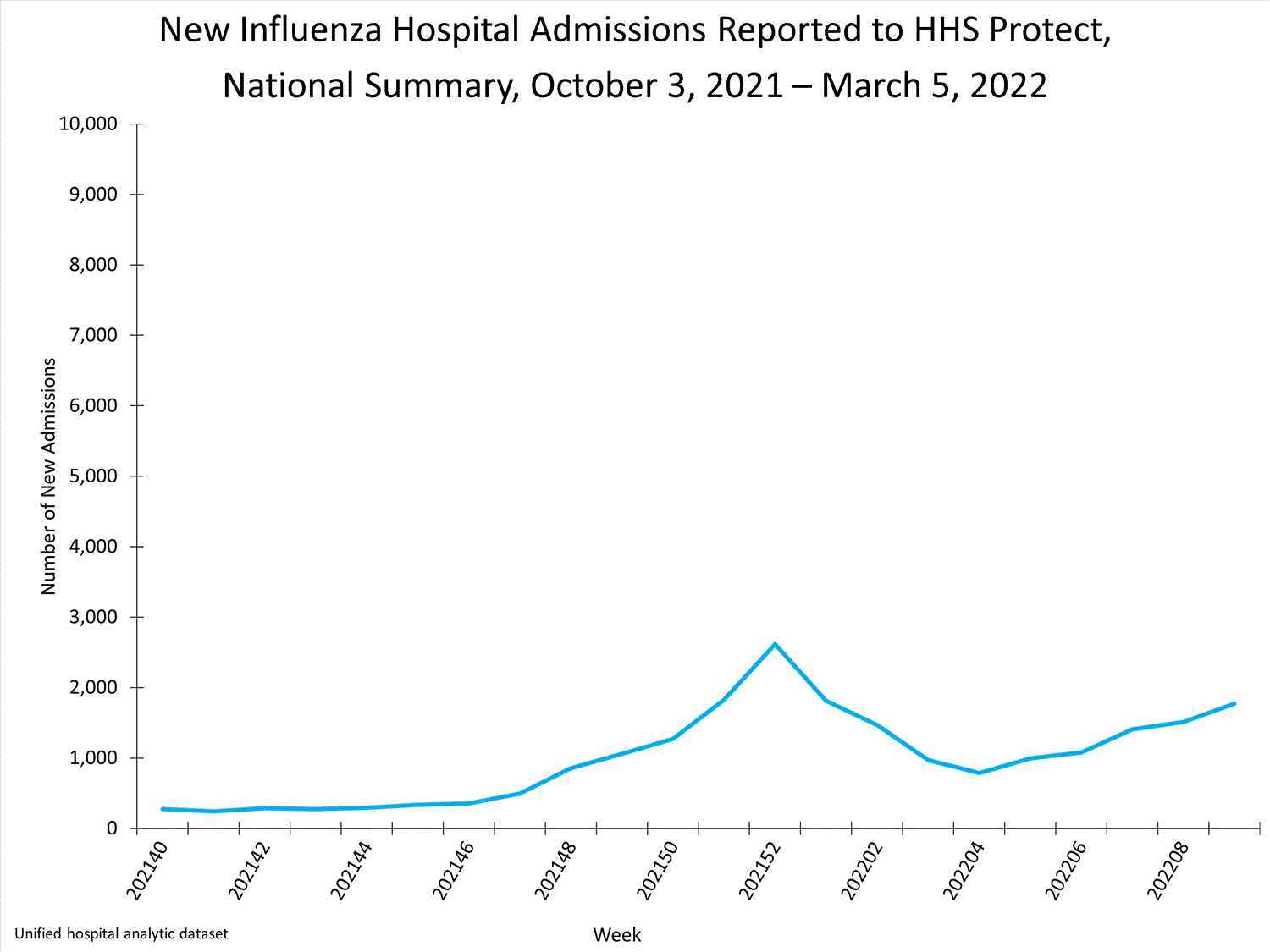
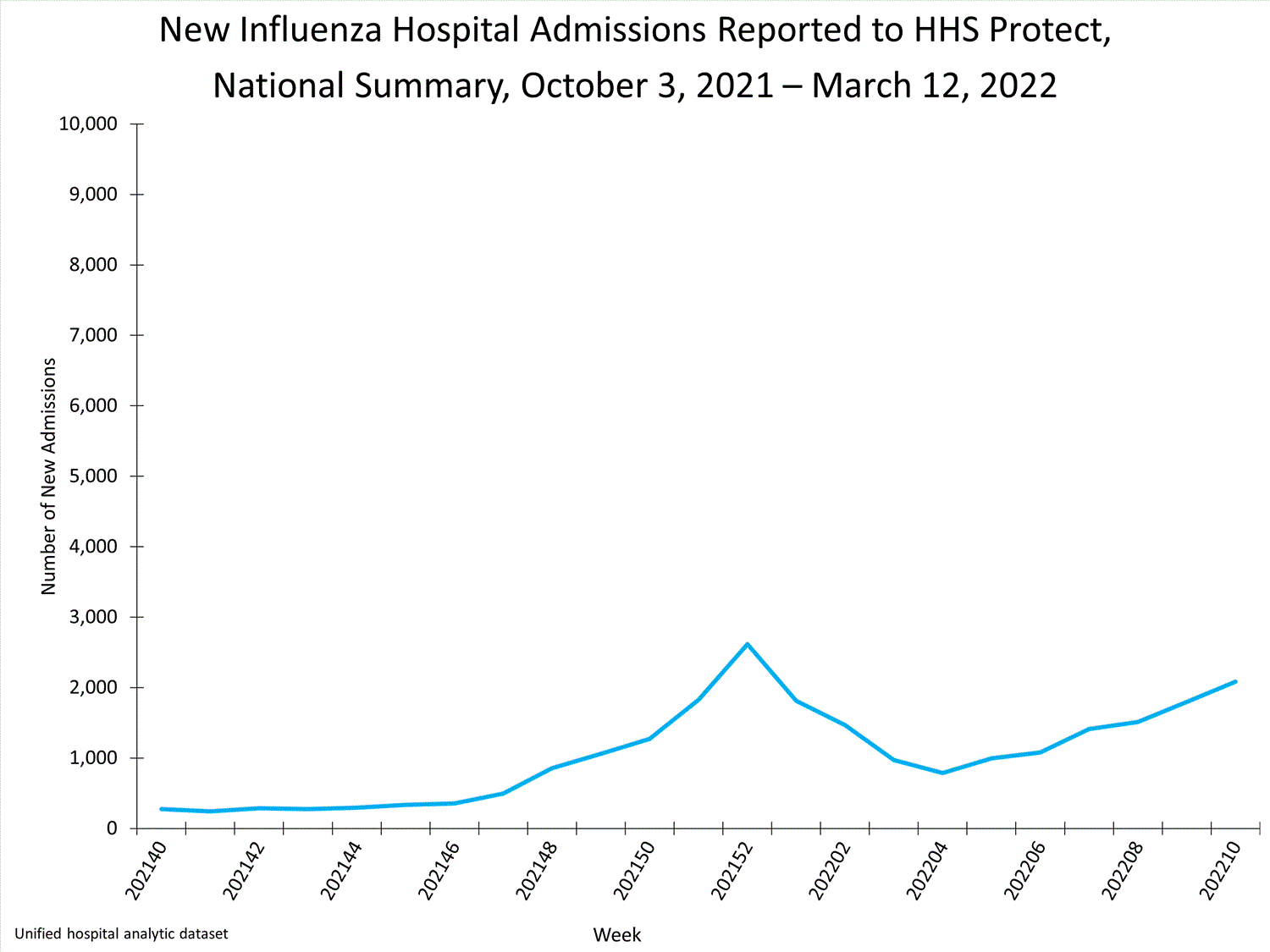
HHS-Protect Hospitalization Surveillance
Hospitals report to HHS-Protect the number of patients admitted with laboratory-confirmed influenza. During week 52, 2,615 patients with laboratory-confirmed influenza were admitted to the hospital.
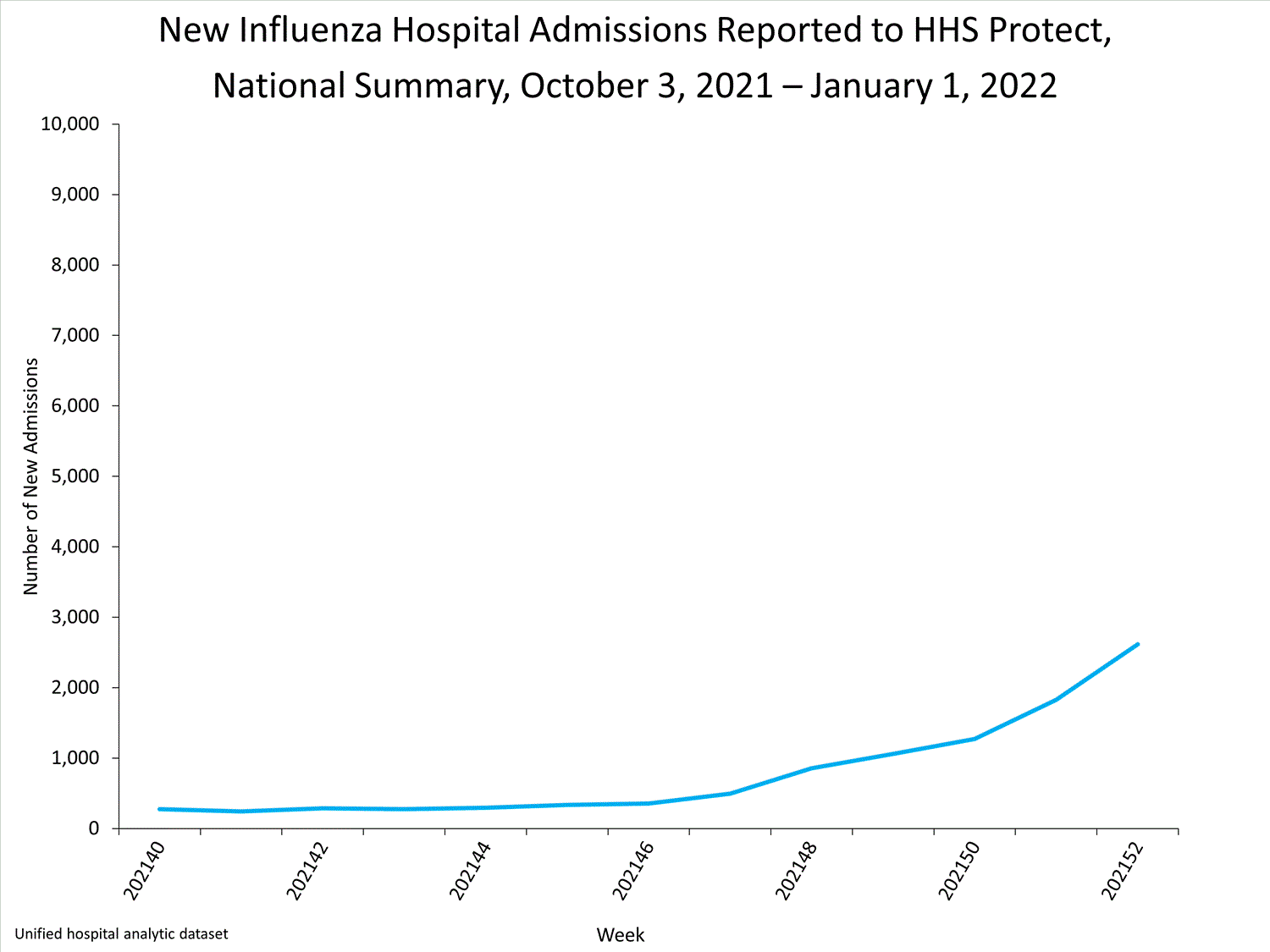
View Chart Dataexcel icon | View Full Screen
Additional HHS Protect hospitalization surveillance information:
Surveillance Methods | Additional Dataexternal icon
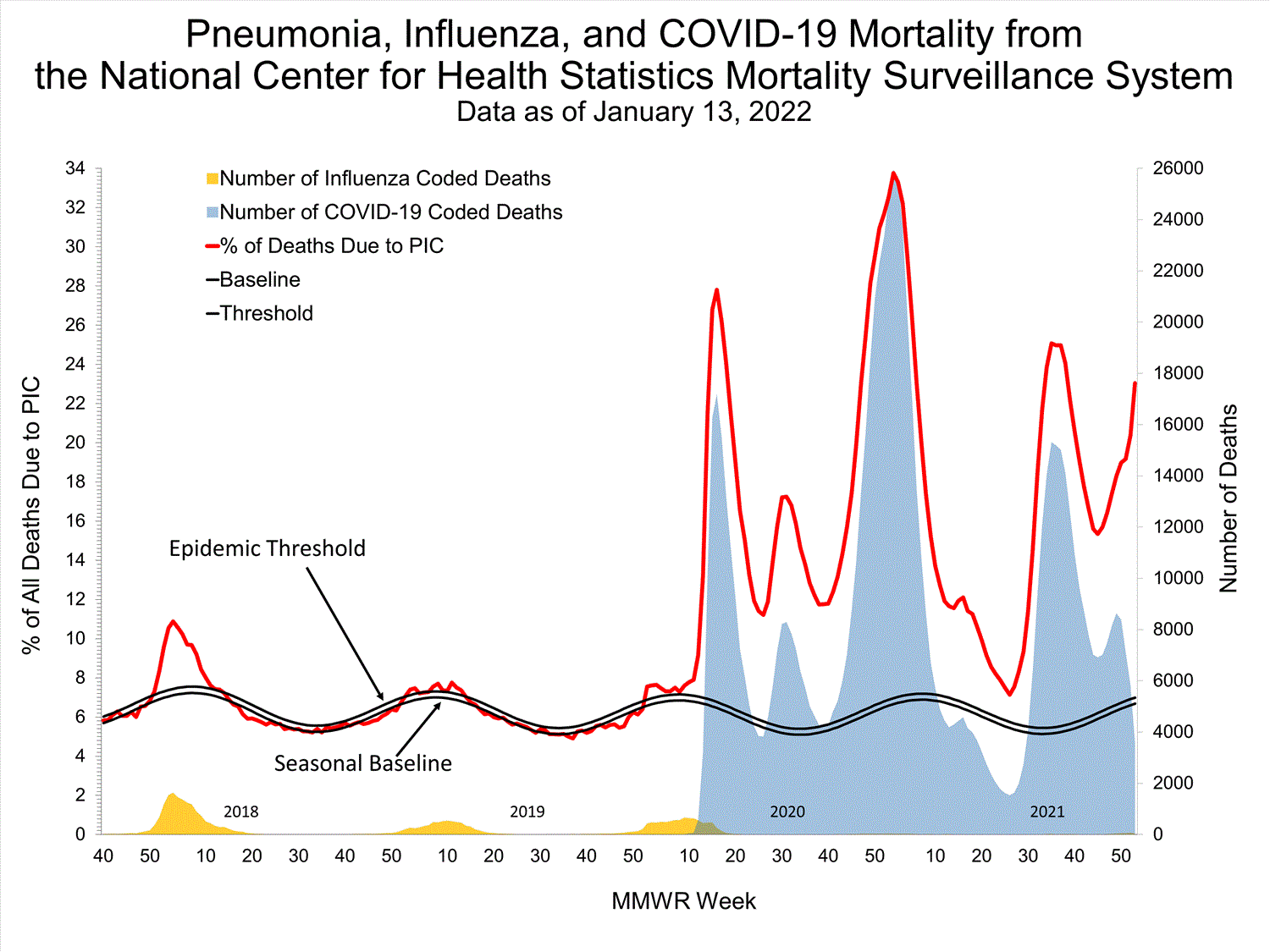
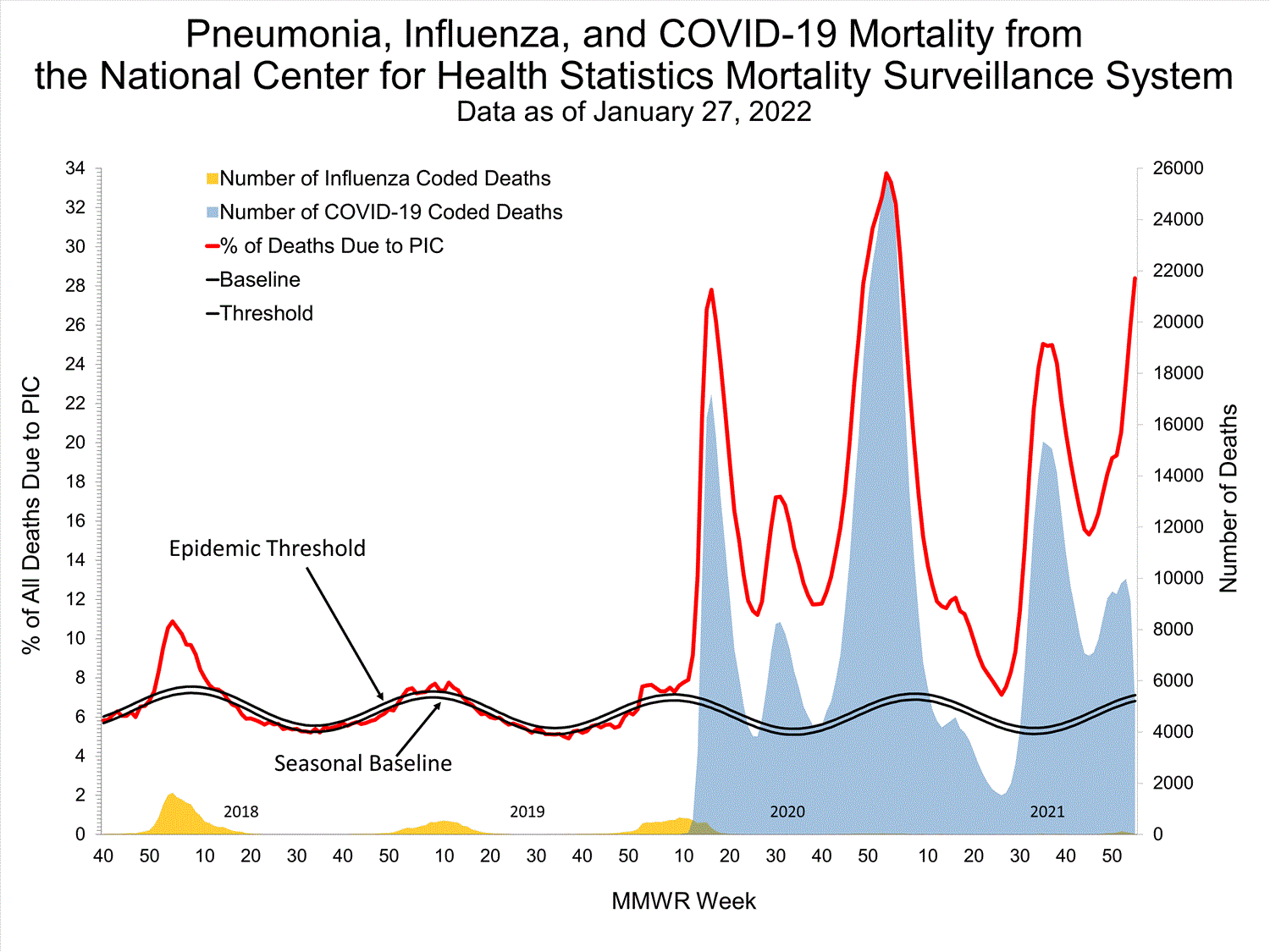
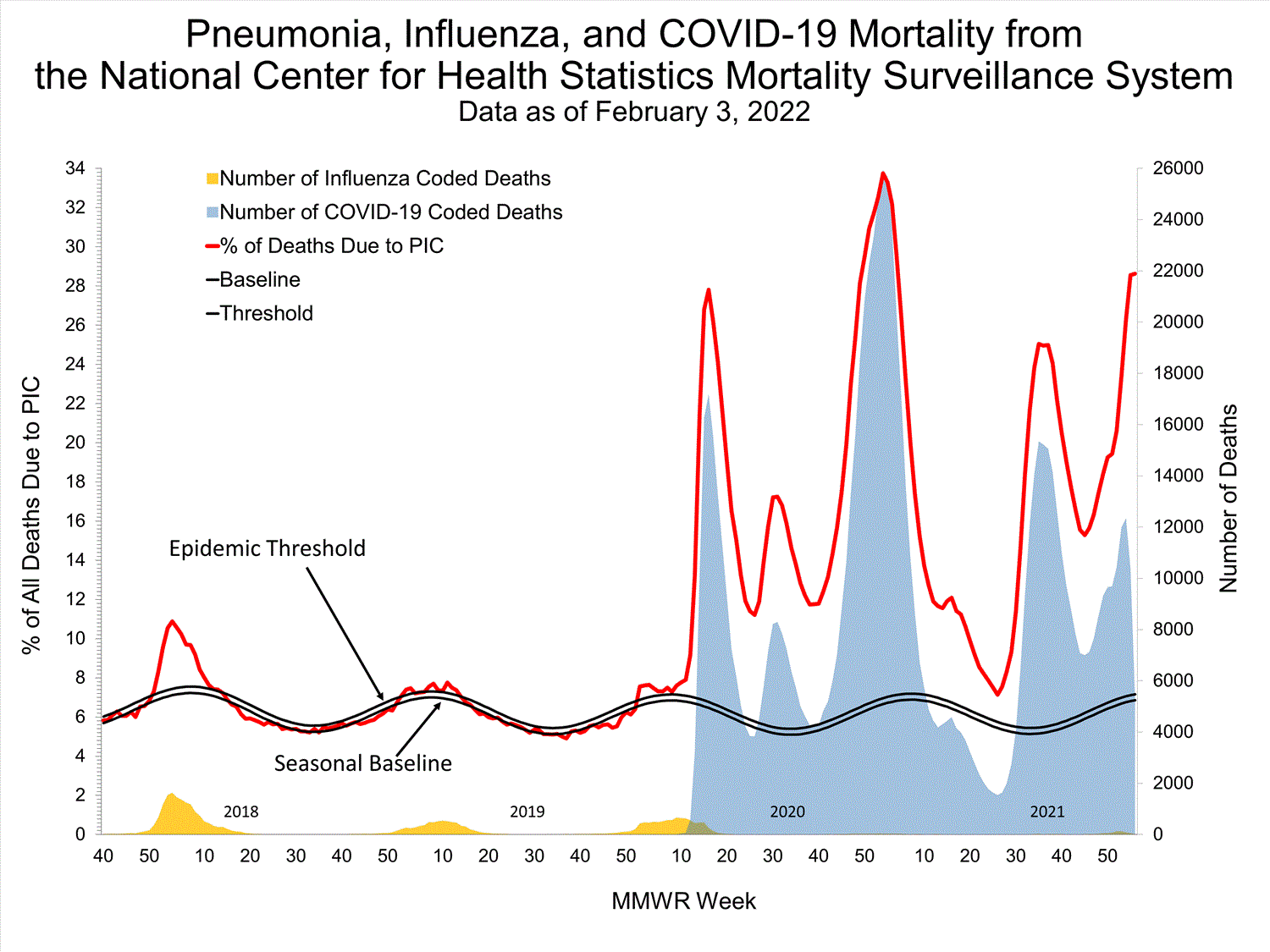
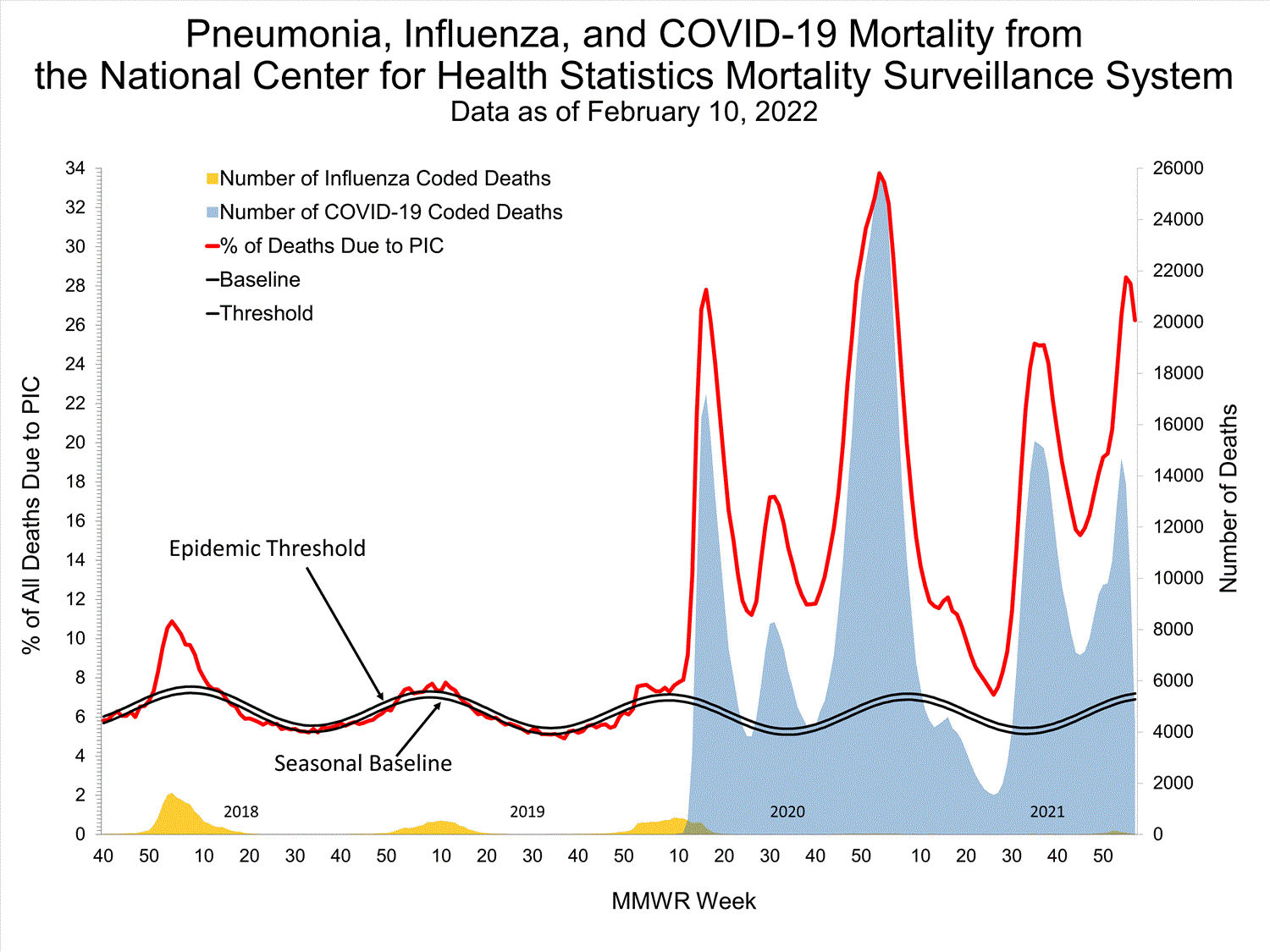
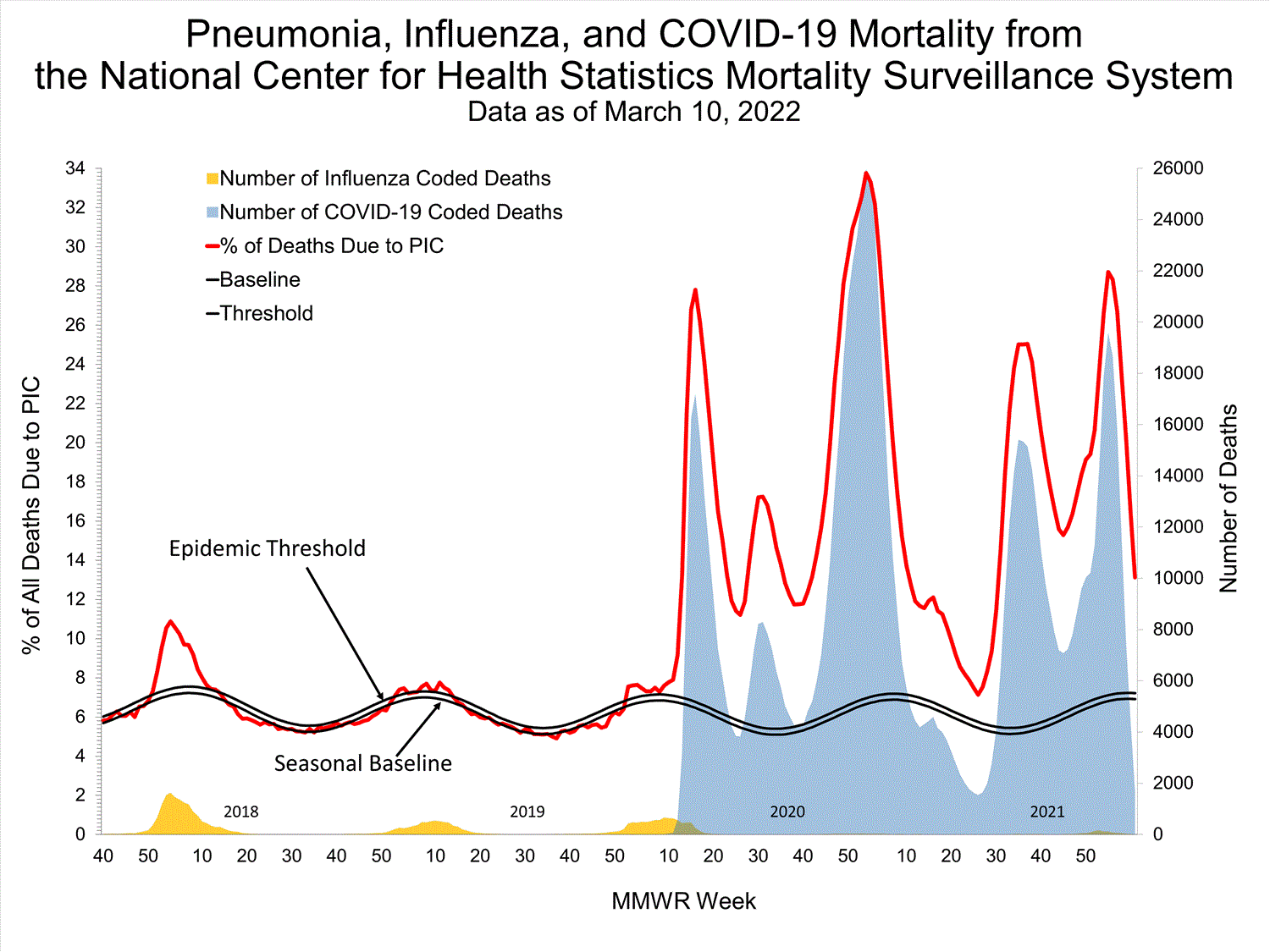
Mortality Surveillance
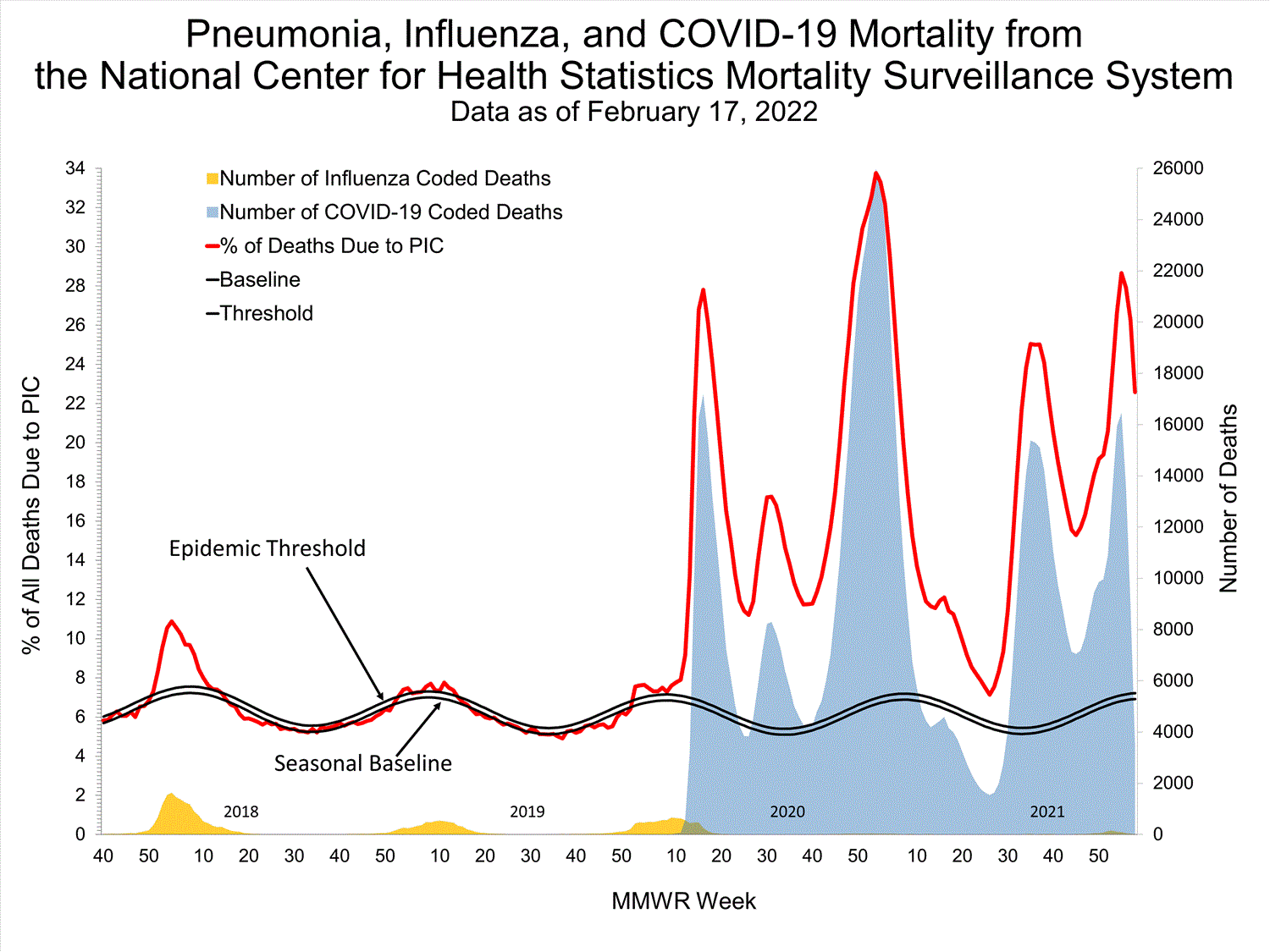
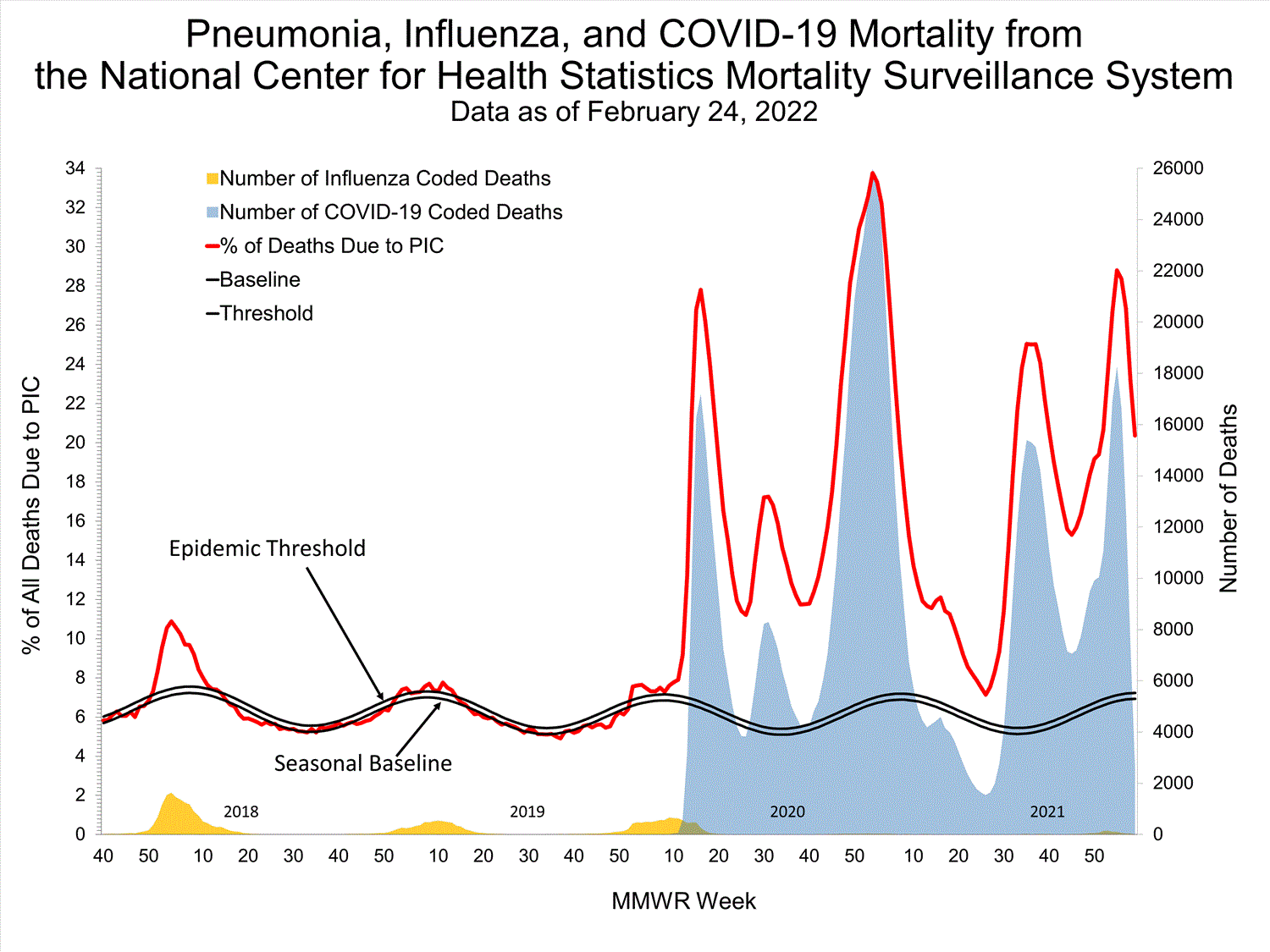
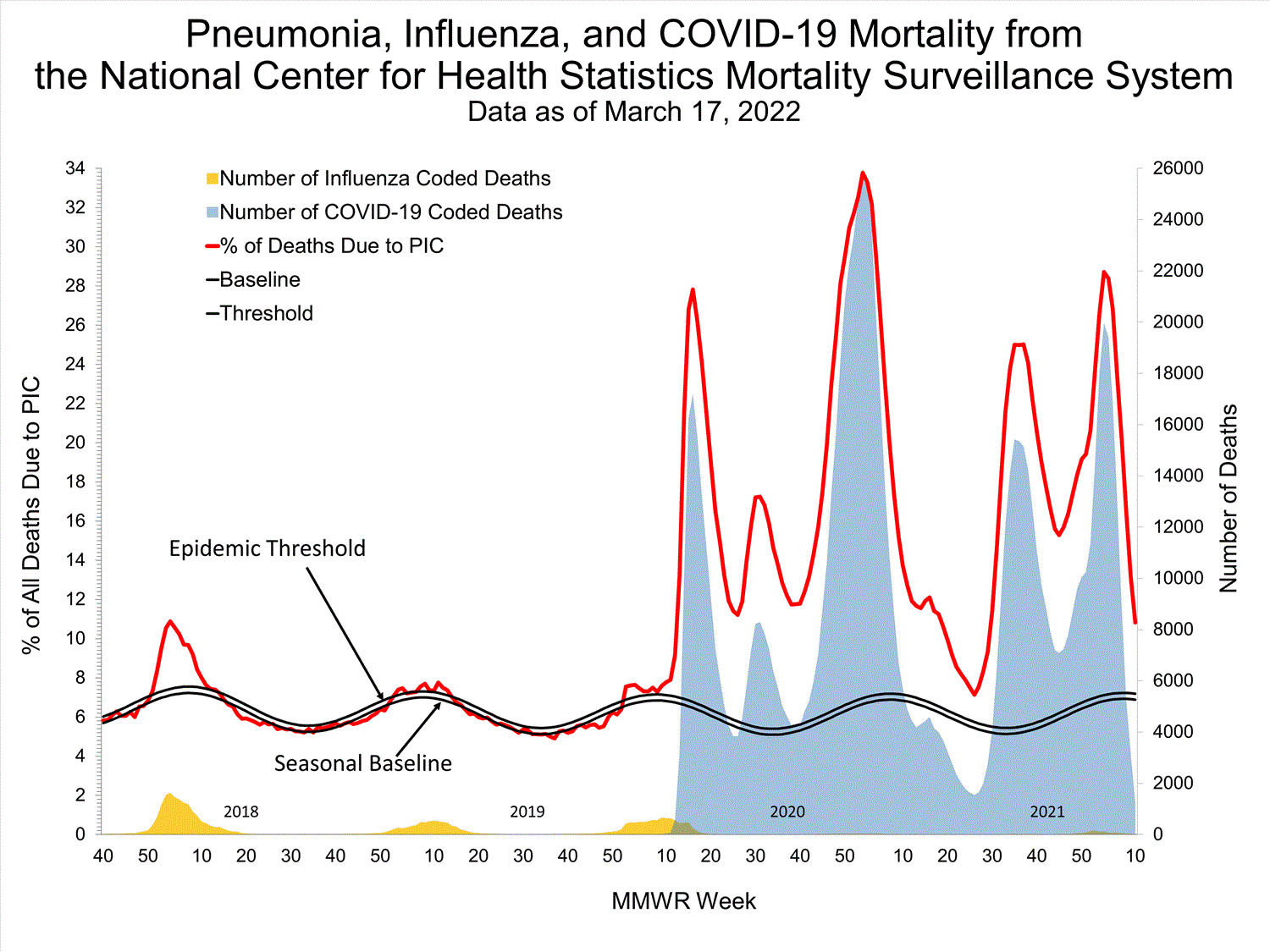
National Center for Health Statistics (NCHS) Mortality Surveillance
Based on NCHS mortality surveillance data available on January 6, 2022, 19.9% of the deaths that occurred during the week ending January 1, 2022 (week 52), were due to pneumonia, influenza, and/or COVID-19 (PIC). This percentage is above the epidemic threshold of 6.9% for this week. Among the 3,252 PIC deaths reported for this week, 2,519 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, and 31 listed influenza, indicating that current PIC mortality is due primarily to COVID-19 and not influenza. The data presented are preliminary and may change as more data are received and processed.
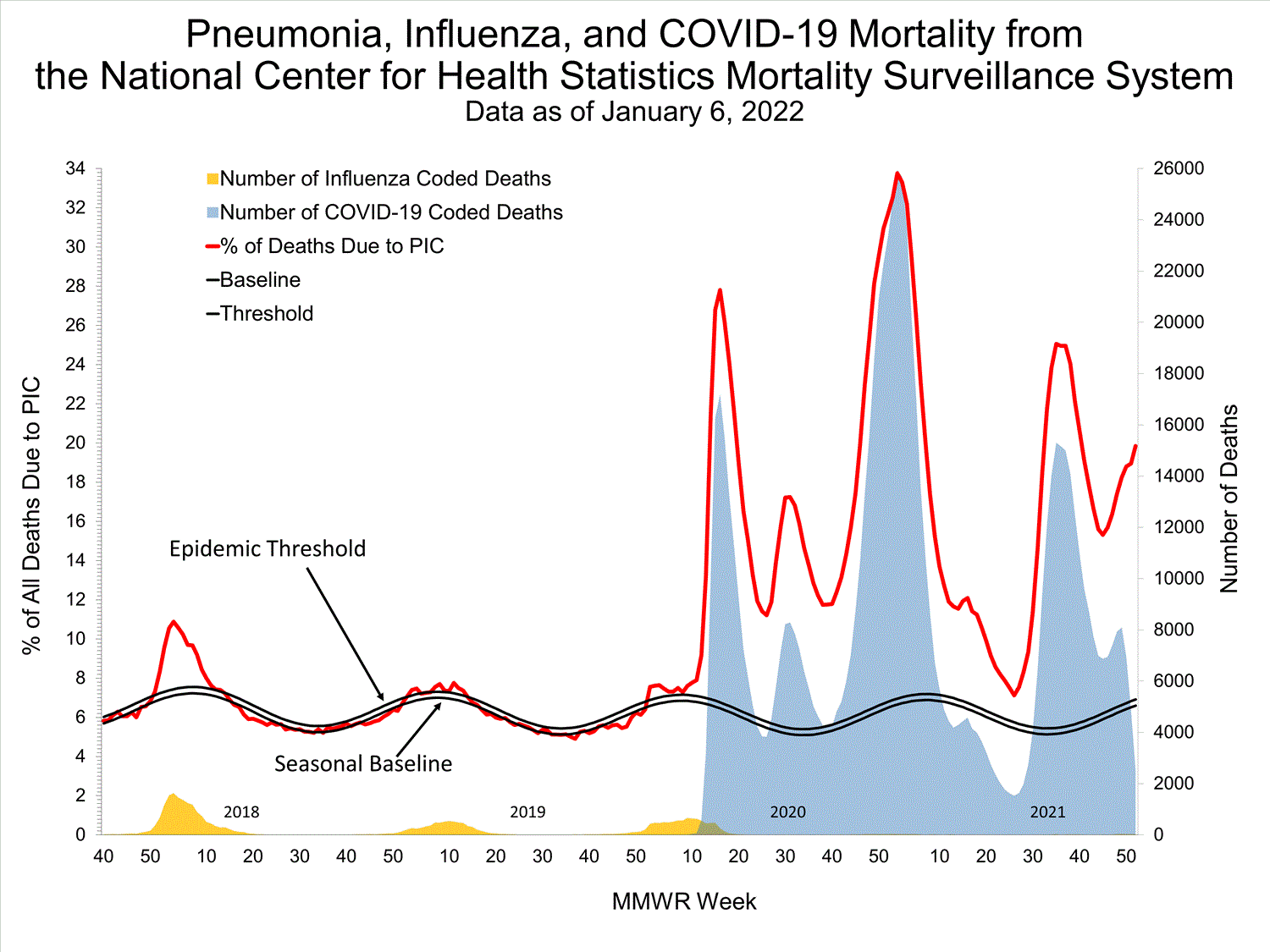
View Chart Dataexcel icon | View Full Screen
Additional pneumonia, influenza and COVID-19 mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
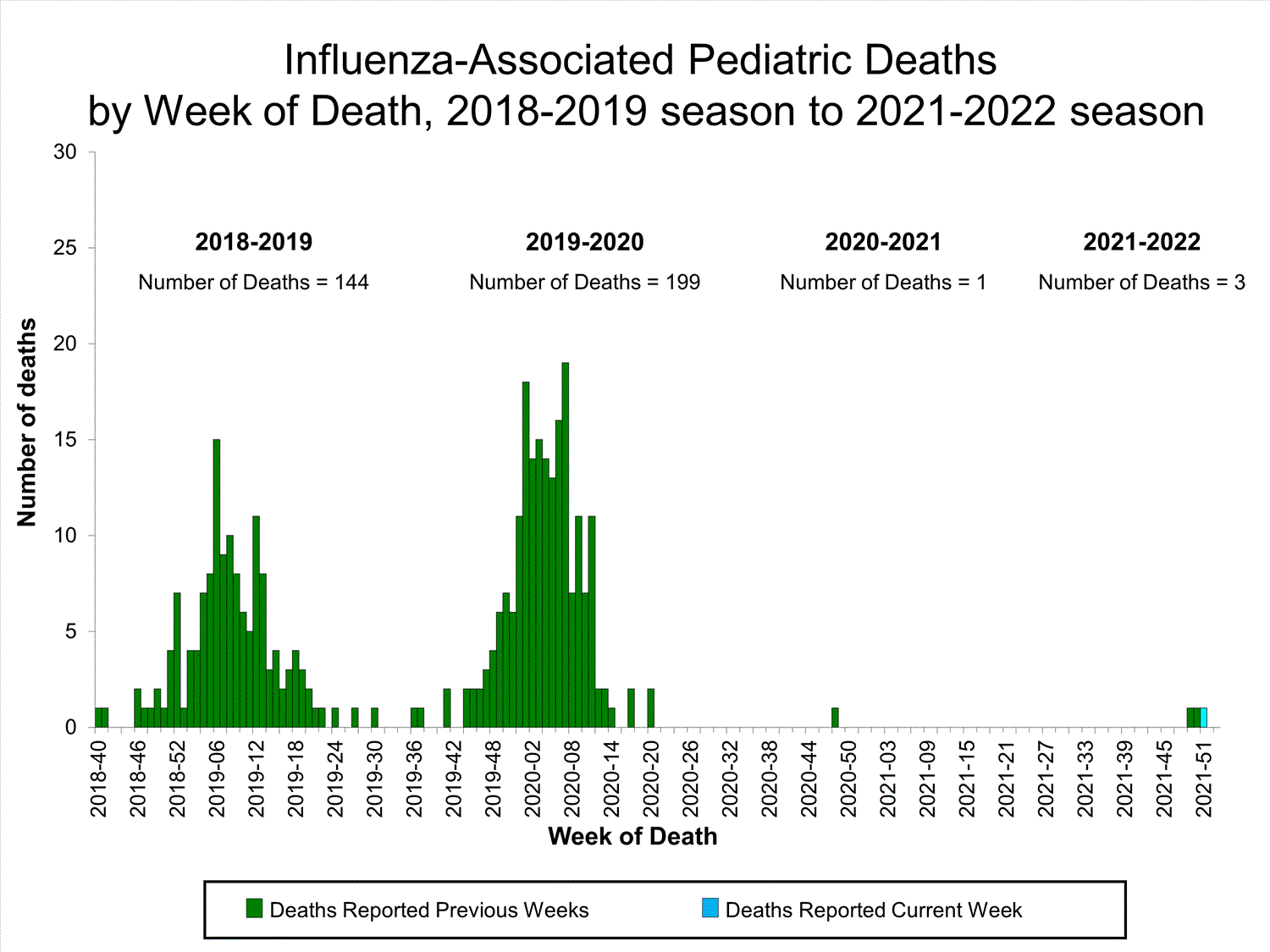
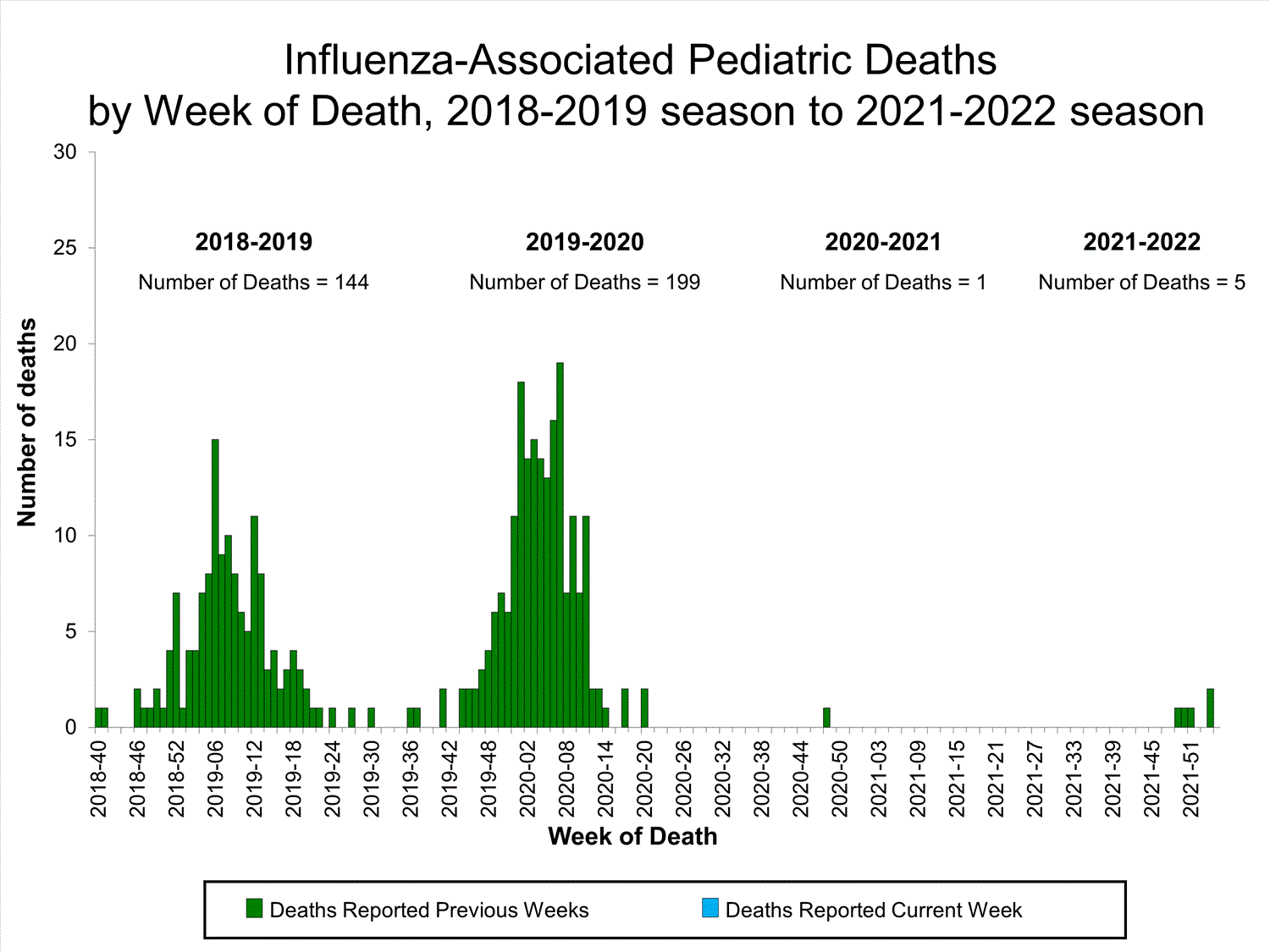
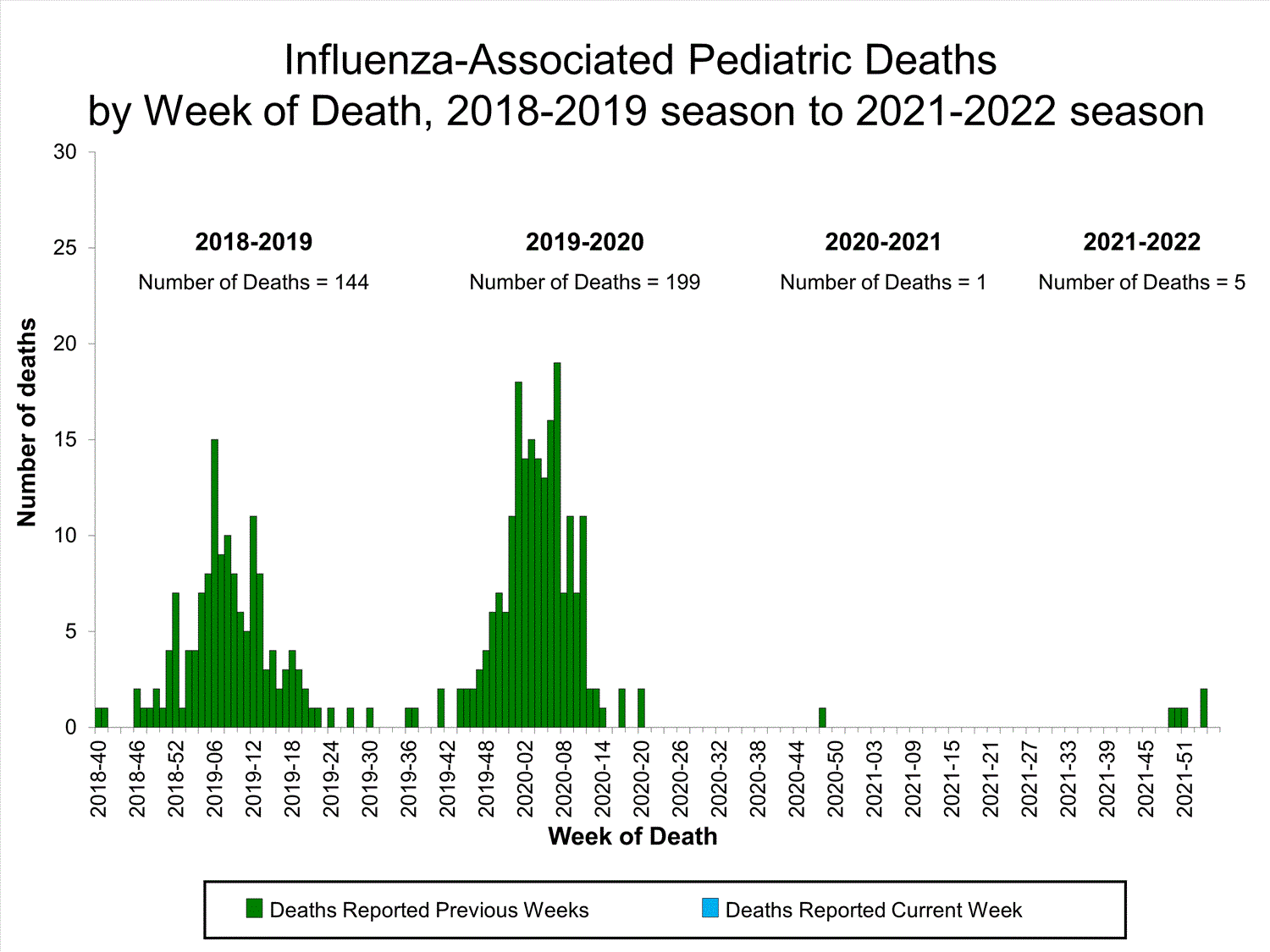
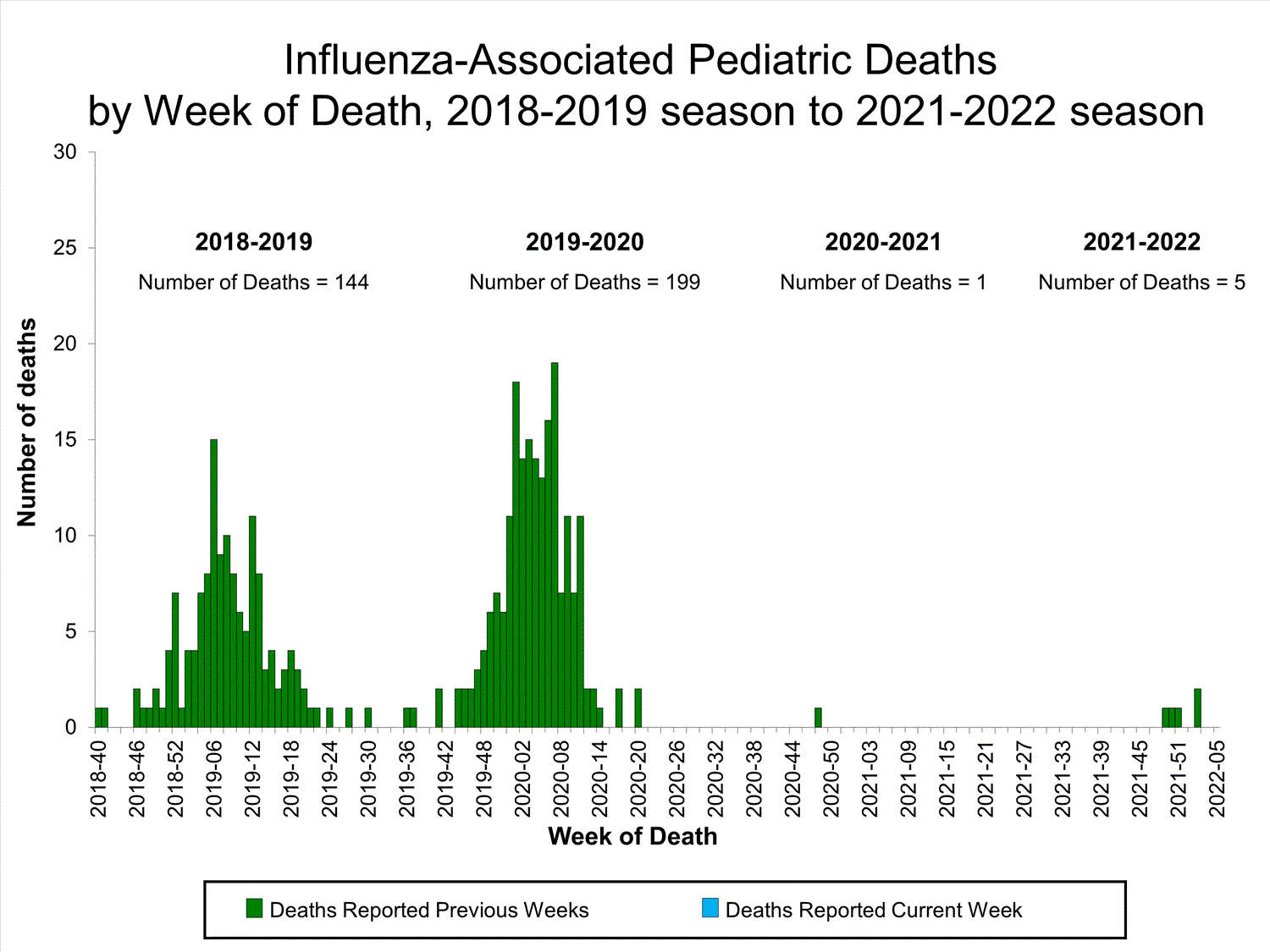
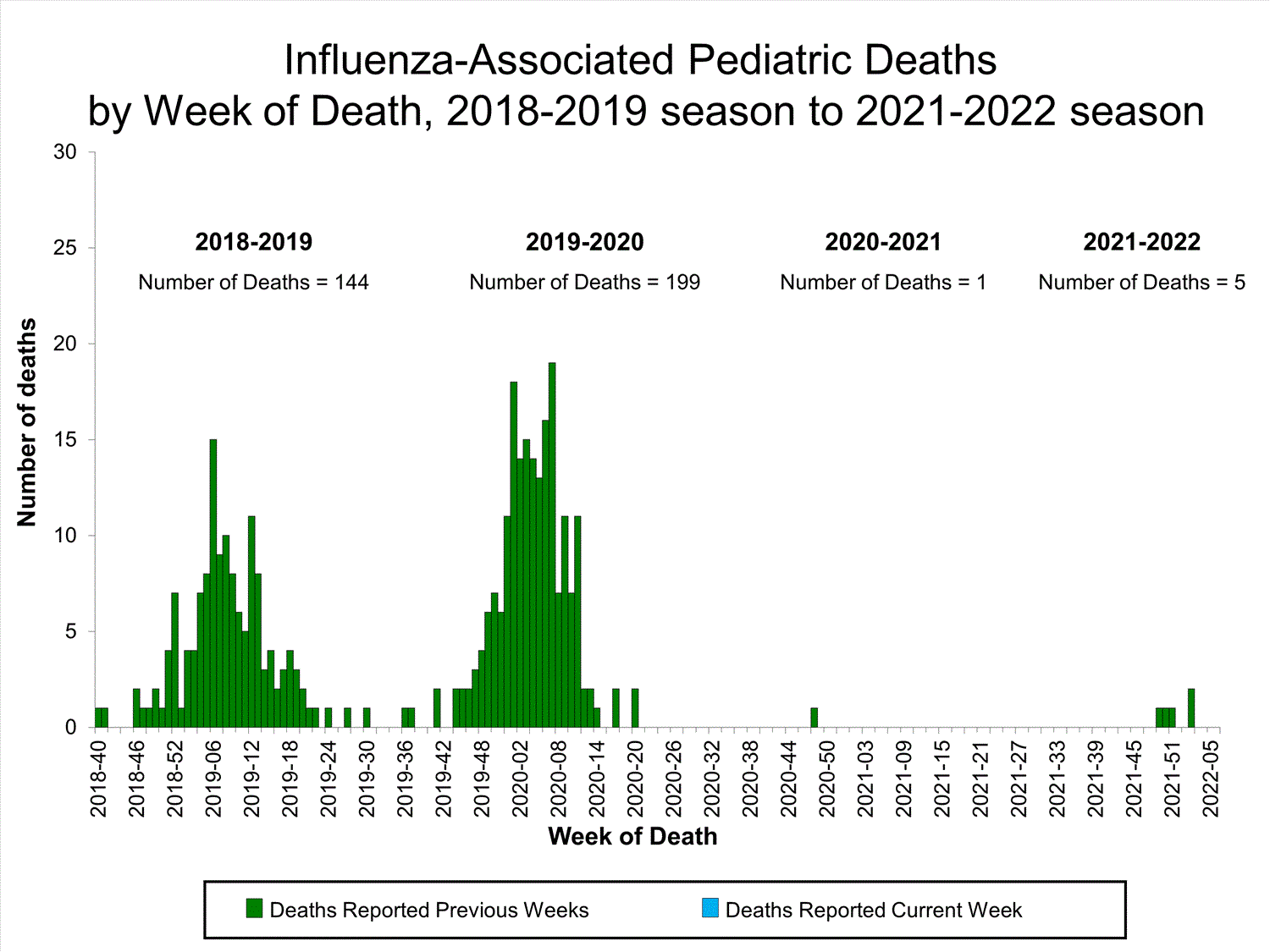
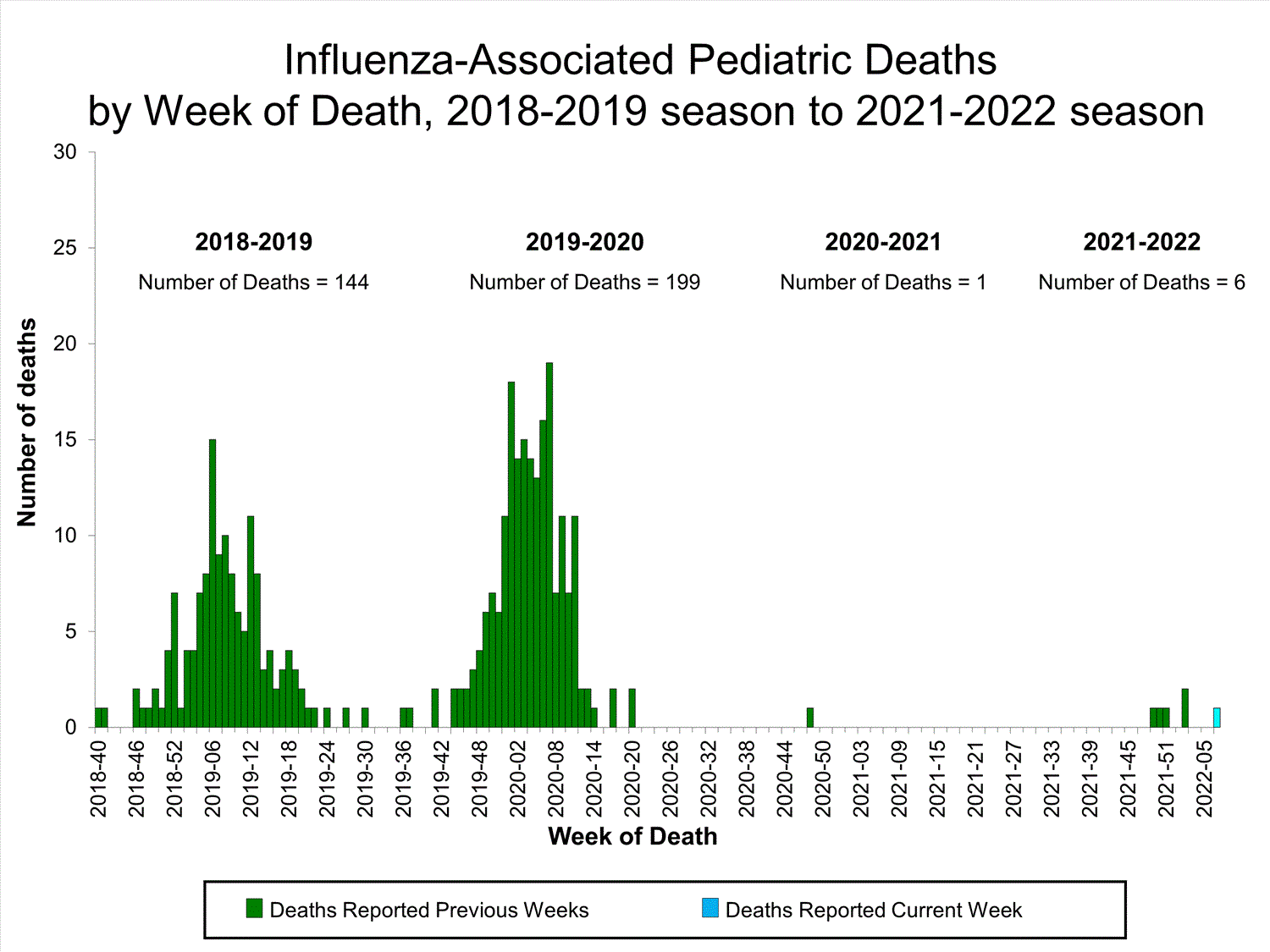
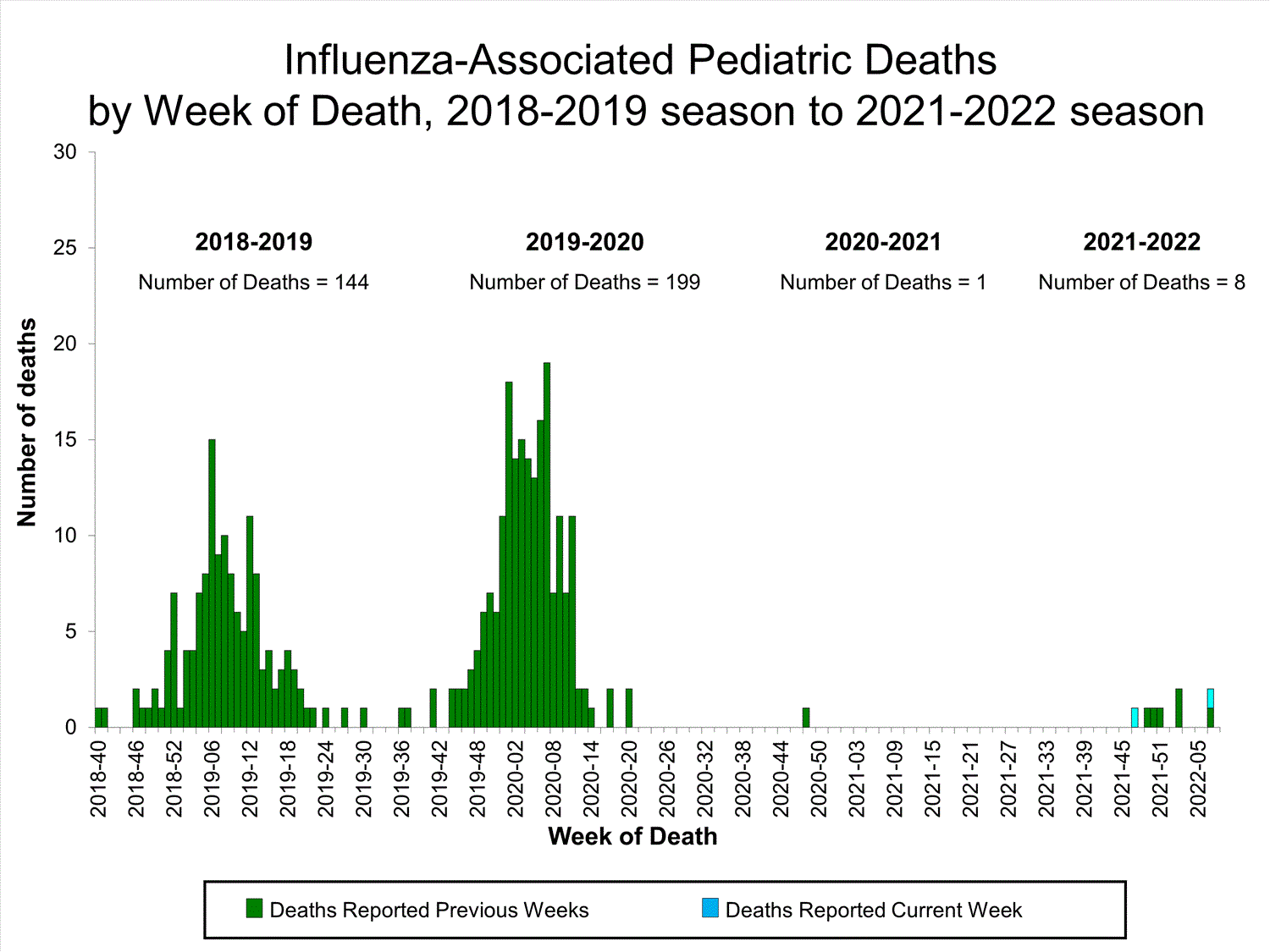
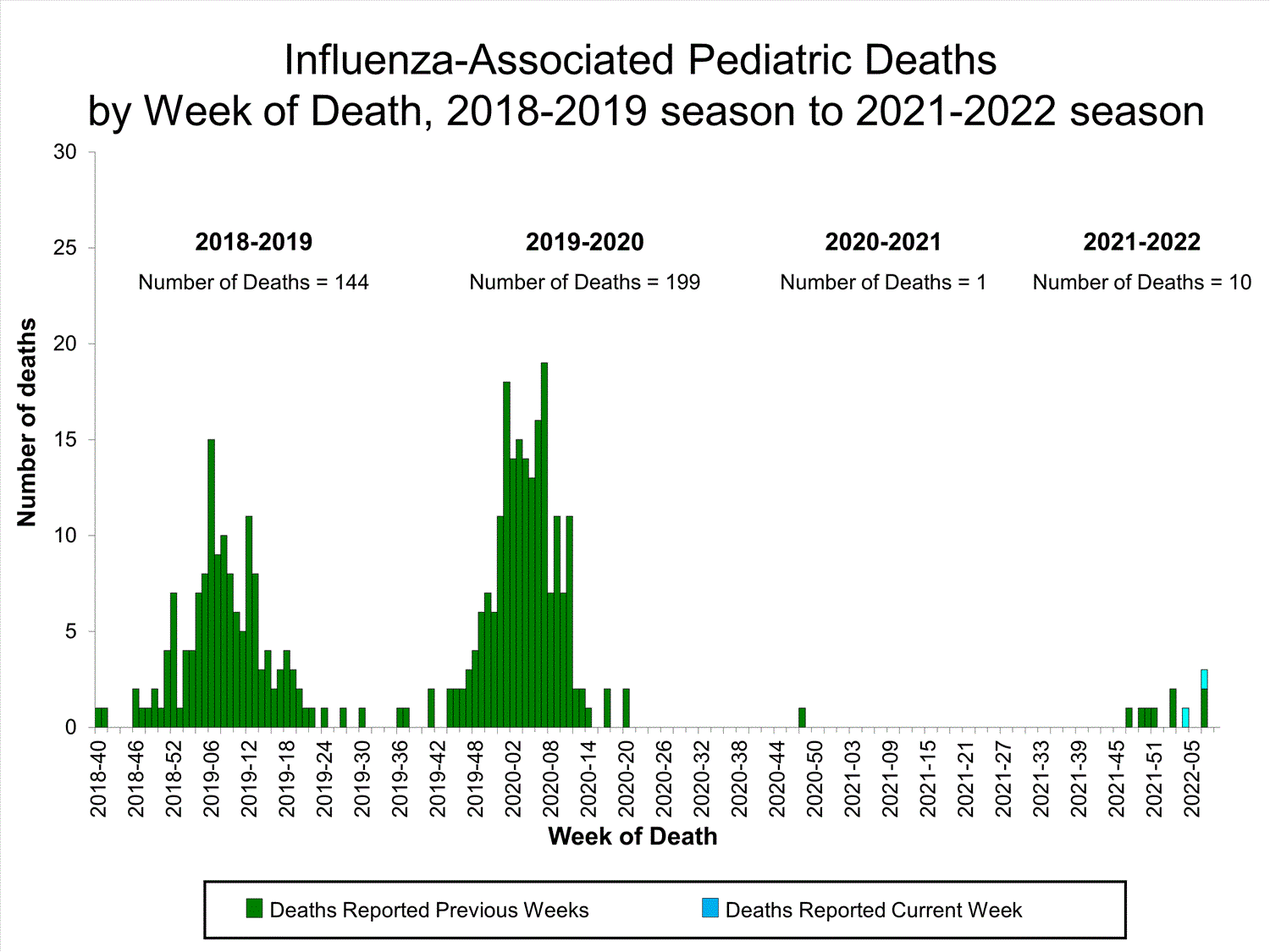
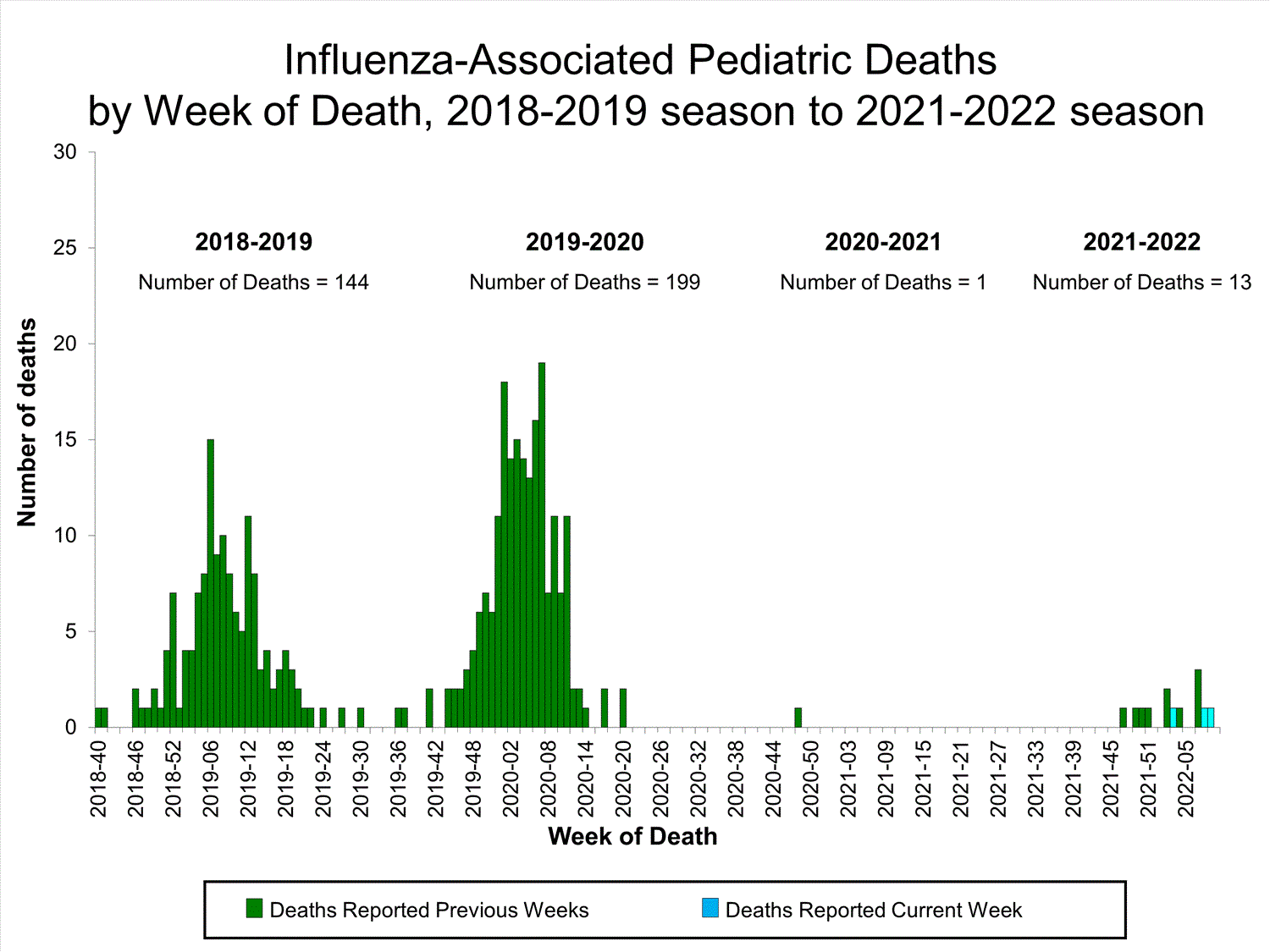
Influenza-Associated Pediatric Mortality
No influenza-associated pediatric deaths were reported to CDC during week 52.
A total of two influenza-associated pediatric deaths occurring during the 2021-2022 season have been reported to CDC.
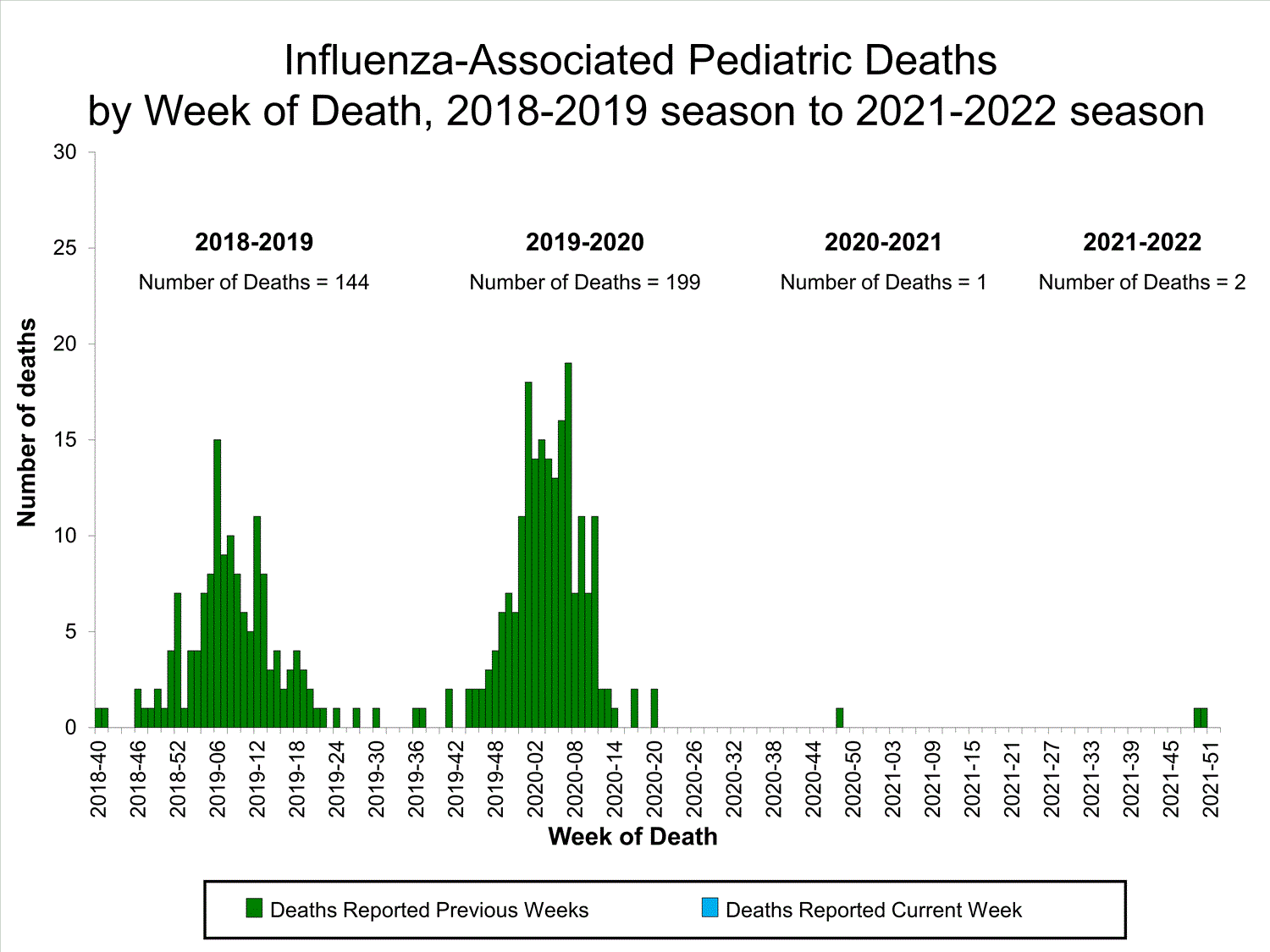 View Full Screen
View Full Screen Additional pediatric mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics.
National Institute for Occupational Safety and Health: Monthly surveillance data on the prevalence of health-related workplace absenteeism among full-time workers in the United States are available from NIOSH.
U.S. State and local influenza surveillance: Select a jurisdiction below to access the latest local influenza information.
World Health Organization:
Additional influenza surveillance information from participating WHO member nations is available through
FluNetexternal icon and the Global Epidemiology Reports.external icon
WHO Collaborating Centers for Influenza:
Australiaexternal icon, Chinaexternal icon, Japanexternal icon, the United Kingdomexternal icon, and the United States (CDC in Atlanta, Georgia)
Europe:
The most up-to-date influenza information from Europe is available from WHO/Europe and the European Centre for Disease Prevention and Controlexternal icon.
Public Health Agency of Canada:
The most up-to-date influenza information from Canada is available in Canada’s weekly FluWatch reportexternal icon.
Public Health England:
The most up-to-date influenza information from the United Kingdom is available from Public Health Englandexternal icon.
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component is available on the surveillance methods page.
Page last reviewed: January 7, 2022, 11:00 AM

Comment