From: "Media@cdc.gov (CDC)"
To: MMWR-MEDIA@LISTSERV.CDC.GOV
Subject: CDC Press Release: More birth defects seen in parts of U.S. with local Zika spread *Embargoed Until 1 PM ET Thursday, January 25, 2018*
Date: Jan 25, 2018 11:35 AM
Attachments: mm6703-H.PDF
To: MMWR-MEDIA@LISTSERV.CDC.GOV
Subject: CDC Press Release: More birth defects seen in parts of U.S. with local Zika spread *Embargoed Until 1 PM ET Thursday, January 25, 2018*
Date: Jan 25, 2018 11:35 AM
Attachments: mm6703-H.PDF
| Press Release Embargoed Until 1 PM ET Thursday, January 25, 2018 Contact: CDC Media Relations (404) 639-3286 More birth defects seen in parts of U.S. with local Zika spread Findings show need for strong birth-defect surveillance networks Birth defects most strongly linked to Zika virus infection during pregnancy have increased in parts of the United States that have had local Zika virus transmission, according to a report in CDC?s Morbidity and Mortality Weekly Report (MMWR).Areas with local transmission of Zika ? southern Florida, a portion of south Texas, and Puerto Rico ? saw a 21 percent increase in births with outcomes most strongly linked to Zika virus in the last half of 2016 compared with births in the first half of that year. It is not known if this increase is due to local transmission of Zika virus alone, or if there are other contributing factors. Most of the mothers who had babies with the Zika virus-linked birth defects did not have laboratory evidence of Zika virus infection?either because they were not tested, were not tested at the right time, or were not exposed to Zika virus. All cases with birth defects potentially related to Zika virus infection are monitored by birth defects surveillance systems. ?Babies with Zika-related birth defects need all the help they can get, as soon as possible and for as long as they need it,? said CDC Director Brenda Fitzgerald, M.D. ?This report highlights the critical importance of documenting birth defects possibly related to Zika and our need to maintain vigilance.? CDC looked at nearly 1 million births in 2016 in 15 U.S. states and territories, including Florida (select southern counties), Georgia (select metro-Atlanta counties), Hawaii, Iowa, Illinois, Massachusetts, New Jersey, New York (excluding New York City), North Carolina (select regions), Puerto Rico, Rhode Island, South Carolina, Texas (select regions), Utah, and Vermont. About three out of every 1,000 babies born in 15 states and territories in 2016 had a birth defect possibly associated with Zika virus infection in the mother:
Because many pregnant women exposed to Zika virus in late 2016 gave birth in 2017, CDC researchers anticipate that there could be another increase in possible Zika-related birth defects when 2017 data are analyzed. CDC uses tracking systems to find birth defects that might be related to Zika virus:
For more information about Zika virus and pregnancy visit www.cdc.gov/pregnancy/zika. |

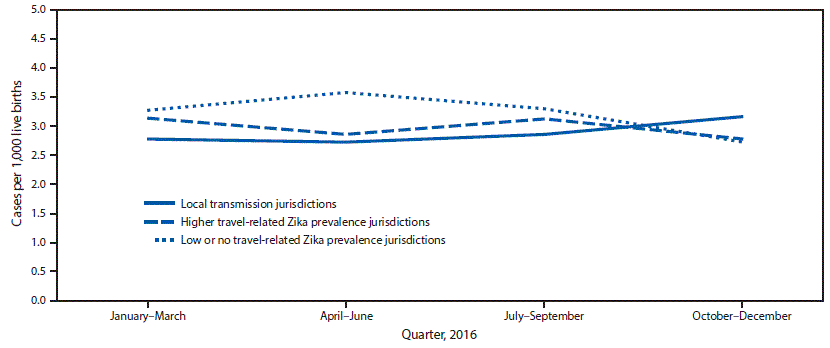 * Local transmission jurisdictions included Florida (selected southern counties), Puerto Rico, and Texas (Public Health Region 11).
* Local transmission jurisdictions included Florida (selected southern counties), Puerto Rico, and Texas (Public Health Region 11).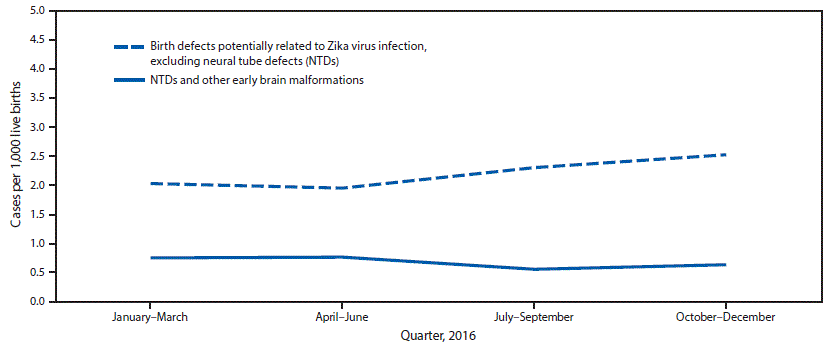 *Fetuses and infants were aggregated into the following four mutually exclusive categories: those with 1) brain abnormalities with or without microcephaly (head circumference at delivery <3rd percentile for sex and gestational age); 2) NTDs and other early brain malformations; 3) eye abnormalities among those without mention of a brain abnormality included in the first two categories; and 4) other consequences of central nervous system dysfunction, specifically joint contractures and congenital sensorineural deafness, among those without mention of brain or eye abnormalities included in another category.
*Fetuses and infants were aggregated into the following four mutually exclusive categories: those with 1) brain abnormalities with or without microcephaly (head circumference at delivery <3rd percentile for sex and gestational age); 2) NTDs and other early brain malformations; 3) eye abnormalities among those without mention of a brain abnormality included in the first two categories; and 4) other consequences of central nervous system dysfunction, specifically joint contractures and congenital sensorineural deafness, among those without mention of brain or eye abnormalities included in another category.
Comment