http://okeefe-lab.blogspot.co.uk/201...v-gag.html?m=1
http://www.newmicrobiologica.org/PUB...2012/3/341.pdf
http://www.newmicrobiologica.org/PUB...2012/3/341.pdf
XMRV and polytropic MLV-related virus have been controversially associated with chronic fatigue syndrome (CFS).
Subsequent reports failed to detect XMRV and MLV-related virus in CFS patients, and the previous results have been
interpreted as a massive laboratory contamination by mouse DNA sequences. Among 12 sequential CFS patients, two
were positive for XMRV/MLV sequences. In contrast, 40 selected control subjects were negative. CSF patients and
controls were negative for mitochondrial mouse-specific DNA sequences. These findings do not confirm the high frequency
of MLV-related viruses infection in CFS patients, but also contrast the widespread laboratory contamination
previously suggested.
Key words: Chronic fatigue syndrome, XMRV, MLV-related virus, Polytropic MLV, Ecotropic MLV.
Subsequent reports failed to detect XMRV and MLV-related virus in CFS patients, and the previous results have been
interpreted as a massive laboratory contamination by mouse DNA sequences. Among 12 sequential CFS patients, two
were positive for XMRV/MLV sequences. In contrast, 40 selected control subjects were negative. CSF patients and
controls were negative for mitochondrial mouse-specific DNA sequences. These findings do not confirm the high frequency
of MLV-related viruses infection in CFS patients, but also contrast the widespread laboratory contamination
previously suggested.
Key words: Chronic fatigue syndrome, XMRV, MLV-related virus, Polytropic MLV, Ecotropic MLV.




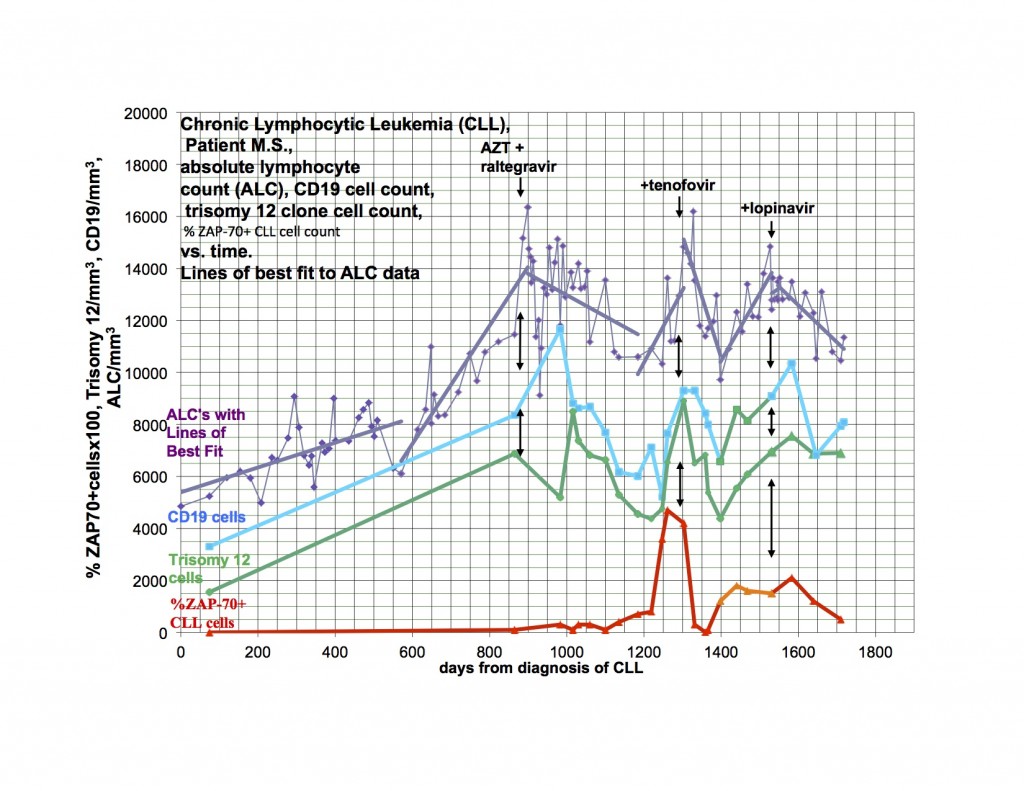


Comment