Source: http://wwwnc.cdc.gov/eid/article/21/5/14-1181_article
Volume 21, Number 5?May 2015
Letter
Outbreak of Leishmania braziliensis Cutaneous Leishmaniasis, Sa?l, French Guiana
On This Page
Figures
Downloads
Suggested citation for this article
To the Editor: New World cutaneous leishmaniasis (CL), a zoonotic disease, is increasingly seen among travelers returning from Latin American countries, particularly from Bolivia, Belize, and French Guiana (1). The epidemiology of CL in the Americas is heterogeneous and has complex variations in transmission cycles, reservoir hosts, and sandfly vectors. Changing human activities that affect these factors may have resulted in the emergence of species with distinct pathogenic potentials and responses to therapy. In the Guianan ecoregion complex, leishmaniasis is endemic, and 5 coexisting Leishmania parasite species are known to infect humans: L. guyanensis, L. braziliensis, L. amazonensis, L. naiffi, and L. lainsoni. Among these species, L. guyanensis accounts for ≈85% of CL cases (2).
We report an outbreak of 7 cases of L. braziliensis CL that occurred among 24 scientists who participated in a field mission at Limonade Creek in Sa?l, French Guiana, during October 10?25, 2013. Sa?l is an isolated village in the Amazonian rainforest (3?55′18′′N, 53?18′02′′W).
Among the 7 patients, 6 were male; mean age was 32 ? 5 years. None of the patients were immunocompromised. The scientists stayed in Sa?l a mean of 17 (range 12?30) days. The mean time to symptom onset after they left Sa?l was 19 (range 0?50) days. The mean number of CL lesions was 2.3 (range 1?5). Lesions were localized mainly on lower limbs (11/14 lesions) but also appeared on upper limbs (2/14 lesions) and ears (1/14 lesions). CL was associated with nodular lymphangitis, adenitis, and superficial phlebitis of the affected limb in 2, 3, and 1 patient, respectively. No patients had mucosal involvement, fever, or decline in general health.
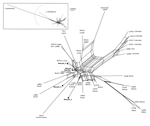 Figure. Data display network showing the genetic diversity of 32 Leishmania braziliensis (according to the multilocus enzyme electrophoresis?based taxonomy) compared with 5 strains from clinical samples (boldface) obtained from visitors to the...
Figure. Data display network showing the genetic diversity of 32 Leishmania braziliensis (according to the multilocus enzyme electrophoresis?based taxonomy) compared with 5 strains from clinical samples (boldface) obtained from visitors to the...
Diagnosis of CL was clinically suggested and confirmed by microscope examination of skin scrapings, which revealed typical amastigotes, by a positive Leishmania species?specific PCR result, or both. L. braziliensis complex was diagnosed by using different molecular techniques, according to the laboratory, and then confirmation of L. braziliensis species was conducted by the French National Reference Center for Leishmaniasis on the basis of a putative translation initiation factor α-subunit gene sequence (3). Leishmania strain genotyping was performed to explore the epidemiology of the implicated strains. Four single-copy genomic loci were amplified from 5 of 7 patient samples; 1 of the samples had a parasite DNA content that was too low to genotype, and 1 was not analyzed. The genetic analysis of the 4 concatenated sequences showed 5 distinct and nonclustered genotypes (Figure). According to local protocols, patients were treated with 20 mg/kg of intramuscular meglumine antimoniate or with 18?38 mg/kg of intravenous liposomal amphotericin B; at publication time, the patients were still being followed.
This outbreak of L. braziliensis CL in French Guiana raises the question of an overall increase in the incidence of this Leishmania species. Until now, outbreaks of L. braziliensis infection have been observed in Argentina, Brazil, Panama, and Venezuela but not Guiana (5?7). In French Guiana, changes in the epidemiology of CL have been observed since 2006; the emergence of L. braziliensis, L. amazonensis, and L. lainsoni infections represented 8.8%, 2.6%, and 1.4%, respectively, of the diagnosed CL cases (8). This trend could be due either to an increase of L. braziliensis prevalence in the forests of Guiana or to a greater presence of humans (e.g., military personnel, scientists, and tourists) in deep forest areas with hot spots of transmission. Favorable environmental conditions in a well-delimited zoonotic microfocus hot spot might have contributed to this high rate of transmission. However the relative genetic diversity of strains we observed among the 5 analyzed patients was unexpected, given the relatively small spatial and temporal scale of the transmission area, and indicates that the reservoirs in this restricted area were infested by distinct genotypes. Development of a peridomestic cycle, perhaps with specific reservoirs (pets) and vectors, cannot be excluded in the Sa?l area.
This case series suggests that caution should be taken in the diagnosis and treatment of CL in patients returning from the Amazonian rainforest, and a species-specific approach based on molecular identification should be proposed to provide appropriate medical management (9). Indeed, although L. braziliensis parasites cause <10% of CL acquired in French Guiana, this species is noteworthy for its involvement of the mucous membranes of the lips, nose, soft palate, or larynx. Also, L. braziliensis parasites usually fail to respond to pentamidine isethionate, the first-line treatment of L. guyanensis CL in French Guiana; instead, treatment of L. braziliensis infection relies on parenteral meglumine antimoniate or liposomal amphotericin B (1).
In summary, the geographic extension of and numeric increase in L. braziliensis cases in the Guiana ecoregion complex, as observed in the rest of South America, are worrisome, and continuous epidemiologic surveillance is needed. Infection with L. braziliensis, which is emerging and has potential to disseminate, must be considered in cases of CL acquired in this region. These issues have key implications for leishmaniasis treatment, which should be directed to the identified species (10).
Guillaume Martin-Blondel, Xavier Iriart, Fouad El Baidouri, St?phane Simon, Deborah Mills, Magalie Demar, Thierry Pistone, Thomas Le Taillandier, Denis Malvy, Jean-Pierre Gangneux, Pierre Couppie, Wendy Munckhof, Bruno Marchou, Christophe Ravel, and Antoine Berry
Author affiliations: Toulouse University Hospital, Toulouse, France (G. Martin-Blondel, X. Iriart, T. Le Taillandier, B. Marchou, A. Berry); INSERM UMR1043, Toulouse, France (G. Martin-Blondel, X. Iriart, A. Berry); French Reference Centre on Leishmaniasis, Montpellier, France (F. El Baidouri, C. Ravel); University of the French West Indies and Guiana, Cayenne, France (S. Simon); Travel Medicine Alliance, Brisbane, Queensland, Australia (D. Mills); Cayenne Hospital, Cayenne (M. Demar, P. Couppie); Bordeaux University Hospital, Bordeaux, France (T. Pistone, D. Malvy); Rennes University Hospital, Rennes, France (J.-P. Gangneux); University of Queensland, Brisbane (W. Munckhof)
Acknowledgment
We thank all the patients who actively participated in this study.
References
Figure
Suggested citation for this article: Martin-Blondel G, Iriart X, El Baidouri F, Simon S, Mills D, Demar M, et al. Outbreak of Leishmania braziliensis cutaneous leishmaniasis, Sa?l, French Guiana [letter]. Emerg Infect Dis. 2015 May [date cited]. http://dx.doi.org/10.3201/eid2105.141181
DOI: 10.3201/eid2105.141181
Related Links
Table of Contents ? Volume 21, Number 5?May 2015
Volume 21, Number 5?May 2015
Letter
Outbreak of Leishmania braziliensis Cutaneous Leishmaniasis, Sa?l, French Guiana
On This Page
Figures
Downloads
Suggested citation for this article
To the Editor: New World cutaneous leishmaniasis (CL), a zoonotic disease, is increasingly seen among travelers returning from Latin American countries, particularly from Bolivia, Belize, and French Guiana (1). The epidemiology of CL in the Americas is heterogeneous and has complex variations in transmission cycles, reservoir hosts, and sandfly vectors. Changing human activities that affect these factors may have resulted in the emergence of species with distinct pathogenic potentials and responses to therapy. In the Guianan ecoregion complex, leishmaniasis is endemic, and 5 coexisting Leishmania parasite species are known to infect humans: L. guyanensis, L. braziliensis, L. amazonensis, L. naiffi, and L. lainsoni. Among these species, L. guyanensis accounts for ≈85% of CL cases (2).
We report an outbreak of 7 cases of L. braziliensis CL that occurred among 24 scientists who participated in a field mission at Limonade Creek in Sa?l, French Guiana, during October 10?25, 2013. Sa?l is an isolated village in the Amazonian rainforest (3?55′18′′N, 53?18′02′′W).
Among the 7 patients, 6 were male; mean age was 32 ? 5 years. None of the patients were immunocompromised. The scientists stayed in Sa?l a mean of 17 (range 12?30) days. The mean time to symptom onset after they left Sa?l was 19 (range 0?50) days. The mean number of CL lesions was 2.3 (range 1?5). Lesions were localized mainly on lower limbs (11/14 lesions) but also appeared on upper limbs (2/14 lesions) and ears (1/14 lesions). CL was associated with nodular lymphangitis, adenitis, and superficial phlebitis of the affected limb in 2, 3, and 1 patient, respectively. No patients had mucosal involvement, fever, or decline in general health.
 Figure. Data display network showing the genetic diversity of 32 Leishmania braziliensis (according to the multilocus enzyme electrophoresis?based taxonomy) compared with 5 strains from clinical samples (boldface) obtained from visitors to the...
Figure. Data display network showing the genetic diversity of 32 Leishmania braziliensis (according to the multilocus enzyme electrophoresis?based taxonomy) compared with 5 strains from clinical samples (boldface) obtained from visitors to the...Diagnosis of CL was clinically suggested and confirmed by microscope examination of skin scrapings, which revealed typical amastigotes, by a positive Leishmania species?specific PCR result, or both. L. braziliensis complex was diagnosed by using different molecular techniques, according to the laboratory, and then confirmation of L. braziliensis species was conducted by the French National Reference Center for Leishmaniasis on the basis of a putative translation initiation factor α-subunit gene sequence (3). Leishmania strain genotyping was performed to explore the epidemiology of the implicated strains. Four single-copy genomic loci were amplified from 5 of 7 patient samples; 1 of the samples had a parasite DNA content that was too low to genotype, and 1 was not analyzed. The genetic analysis of the 4 concatenated sequences showed 5 distinct and nonclustered genotypes (Figure). According to local protocols, patients were treated with 20 mg/kg of intramuscular meglumine antimoniate or with 18?38 mg/kg of intravenous liposomal amphotericin B; at publication time, the patients were still being followed.
This outbreak of L. braziliensis CL in French Guiana raises the question of an overall increase in the incidence of this Leishmania species. Until now, outbreaks of L. braziliensis infection have been observed in Argentina, Brazil, Panama, and Venezuela but not Guiana (5?7). In French Guiana, changes in the epidemiology of CL have been observed since 2006; the emergence of L. braziliensis, L. amazonensis, and L. lainsoni infections represented 8.8%, 2.6%, and 1.4%, respectively, of the diagnosed CL cases (8). This trend could be due either to an increase of L. braziliensis prevalence in the forests of Guiana or to a greater presence of humans (e.g., military personnel, scientists, and tourists) in deep forest areas with hot spots of transmission. Favorable environmental conditions in a well-delimited zoonotic microfocus hot spot might have contributed to this high rate of transmission. However the relative genetic diversity of strains we observed among the 5 analyzed patients was unexpected, given the relatively small spatial and temporal scale of the transmission area, and indicates that the reservoirs in this restricted area were infested by distinct genotypes. Development of a peridomestic cycle, perhaps with specific reservoirs (pets) and vectors, cannot be excluded in the Sa?l area.
This case series suggests that caution should be taken in the diagnosis and treatment of CL in patients returning from the Amazonian rainforest, and a species-specific approach based on molecular identification should be proposed to provide appropriate medical management (9). Indeed, although L. braziliensis parasites cause <10% of CL acquired in French Guiana, this species is noteworthy for its involvement of the mucous membranes of the lips, nose, soft palate, or larynx. Also, L. braziliensis parasites usually fail to respond to pentamidine isethionate, the first-line treatment of L. guyanensis CL in French Guiana; instead, treatment of L. braziliensis infection relies on parenteral meglumine antimoniate or liposomal amphotericin B (1).
In summary, the geographic extension of and numeric increase in L. braziliensis cases in the Guiana ecoregion complex, as observed in the rest of South America, are worrisome, and continuous epidemiologic surveillance is needed. Infection with L. braziliensis, which is emerging and has potential to disseminate, must be considered in cases of CL acquired in this region. These issues have key implications for leishmaniasis treatment, which should be directed to the identified species (10).
Guillaume Martin-Blondel, Xavier Iriart, Fouad El Baidouri, St?phane Simon, Deborah Mills, Magalie Demar, Thierry Pistone, Thomas Le Taillandier, Denis Malvy, Jean-Pierre Gangneux, Pierre Couppie, Wendy Munckhof, Bruno Marchou, Christophe Ravel, and Antoine Berry
Author affiliations: Toulouse University Hospital, Toulouse, France (G. Martin-Blondel, X. Iriart, T. Le Taillandier, B. Marchou, A. Berry); INSERM UMR1043, Toulouse, France (G. Martin-Blondel, X. Iriart, A. Berry); French Reference Centre on Leishmaniasis, Montpellier, France (F. El Baidouri, C. Ravel); University of the French West Indies and Guiana, Cayenne, France (S. Simon); Travel Medicine Alliance, Brisbane, Queensland, Australia (D. Mills); Cayenne Hospital, Cayenne (M. Demar, P. Couppie); Bordeaux University Hospital, Bordeaux, France (T. Pistone, D. Malvy); Rennes University Hospital, Rennes, France (J.-P. Gangneux); University of Queensland, Brisbane (W. Munckhof)
Acknowledgment
We thank all the patients who actively participated in this study.
References
- Schwartz E, Hatz C, Blum J. New World cutaneous leishmaniasis in travellers. Lancet Infect Dis. 2006;6:342?9. DOIPubMed
- Desjeux P, Dedet JP. Isoenzyme characterization of 112 Leishmania isolates from French Guiana. Trans R Soc Trop Med Hyg. 1989;83:610?2. DOIPubMed
- El Baidouri F, Diancourt L, Berry V, Chevenet F, Pratlong F, Marty P, Genetic structure and evolution of the Leishmania genus in Africa and Eurasia: what does MLSA tell us. PLoS Negl Trop Dis. 2013;7:e2255. DOIPubMed
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254?67. DOIPubMed
- Krolewiecki AJ, Gil JF, Quipildor M, Cajal SP, Pravia C, Juarez M, Restricted outbreak of American tegumentary leishmaniasis with high microfocal transmission. Am J Trop Med Hyg. 2013;88:578?82. DOIPubMed
- Andrade MS, Brito ME, Silva ST, Ishikawa E, Carvalho SM, Brandao-Filho SP. New outbreak of American tegumentary leishmaniasis in a military training center in the Zona da Mata region, in the north of the State of Pernambuco [in Portuguese]. Rev Soc Bras Med Trop. 2009;42:594?6. DOIPubMed
- Sanchez JL, Diniega BM, Small JW, Miller RN, Andujar JM, Weina PJ, Epidemiologic investigation of an outbreak of cutaneous leishmaniasis in a defined geographic focus of transmission. Am J Trop Med Hyg. 1992;47:47?54 .PubMed
- Carme B, Simon S, Couppie P. Epidemiological survey of leishmaniasis in French Guiana. In: Proceedings of the 3rd French Indies and Guiana Interregional Meeting; 2012 Oct 26?27. Saint-Maurice (France): Institut National de Veille Sanitaire; 2012.
- Lavergne RA, Iriart X, Martin-Blondel G, Chauvin P, Menard S, Fillaux J, Contribution of molecular diagnosis to the management of cutaneous leishmaniasis in travellers. Clin Microbiol Infect. 2014;20:O528?30 . DOIPubMed
- Hodiamont CJ, Kager PA, Bart A, de Vries HJ, van Thiel PP, Leenstra T, Species-directed therapy for leishmaniasis in returning travellers: a comprehensive guide. PLoS Negl Trop Dis. 2014;8:e2832 . DOIPubMed
Figure
Suggested citation for this article: Martin-Blondel G, Iriart X, El Baidouri F, Simon S, Mills D, Demar M, et al. Outbreak of Leishmania braziliensis cutaneous leishmaniasis, Sa?l, French Guiana [letter]. Emerg Infect Dis. 2015 May [date cited]. http://dx.doi.org/10.3201/eid2105.141181
DOI: 10.3201/eid2105.141181
Related Links
Table of Contents ? Volume 21, Number 5?May 2015